Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine
- PMID: 19656878
- PMCID: PMC2753130
- DOI: 10.1128/JVI.01073-09
Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine
Abstract
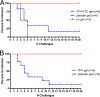
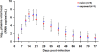
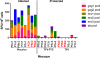
New-generation gels that deliver potent antiretroviral drugs against human immunodeficiency virus type 1 have renewed hopes for topical prophylaxis as a prevention strategy. Previous preclinical research with monkey models suggested that high concentrations and drug combinations are needed for high efficacy. We evaluated two long-acting reverse transcriptase inhibitors, tenofovir (TFV) and emtricitabine (FTC), by using a twice-weekly repeat challenge macaque model and showed that a preexposure vaginal application of gel with 1% TFV alone or in combination with 5% FTC fully protected macaques from a total of 20 exposures to simian-human immunodeficiency virus SF162p3. FTC and TFV were detected in plasma 30 min after vaginal application, suggesting rapid absorption. FTC was detected more frequently than TFV and showed higher levels, reflecting the fivefold-higher concentration of this drug than of TFV. Two of 12 repeatedly exposed but protected macaques showed limited T-cell priming, which did not induce resistance to infection when macaques were rechallenged. Thus, single drugs with durable antiviral activity can provide highly effective topical prophylaxis and overcome the need for noncoital use or for drug combinations which are more complex and costly to formulate and approve.
Figures

Similar articles
-
Durable protection from vaginal simian-human immunodeficiency virus infection in macaques by tenofovir gel and its relationship to drug levels in tissue.J Virol. 2012 Jan;86(2):718-25. doi: 10.1128/JVI.05842-11. Epub 2011 Nov 9. J Virol. 2012. PMID: 22072766 Free PMC article.
-
Prophylactic efficacy of oral emtricitabine and tenofovir disoproxil fumarate combination therapy against a tenofovir-resistant simian/human immunodeficiency virus containing the K65R mutation in macaques.J Infect Dis. 2013 Aug 1;208(3):463-7. doi: 10.1093/infdis/jit189. Epub 2013 Apr 30. J Infect Dis. 2013. PMID: 23633402 Free PMC article.
-
Efficacy of topical tenofovir against transmission of a tenofovir-resistant SHIV in macaques.Retrovirology. 2015 Aug 8;12:69. doi: 10.1186/s12977-015-0195-z. Retrovirology. 2015. PMID: 26253002 Free PMC article.
-
Oral preexposure anti-HIV prophylaxis for high-risk U.S. populations: current considerations in light of new findings.AIDS Patient Care STDS. 2011 Feb;25(2):63-71. doi: 10.1089/apc.2010.0222. AIDS Patient Care STDS. 2011. PMID: 21284497 Review.
-
[Clinical data II. Clinical experience of tenofovir DF in combination with protease inhibitors].Enferm Infecc Microbiol Clin. 2008 Jun;26 Suppl 8:13-8. doi: 10.1157/13126267. Enferm Infecc Microbiol Clin. 2008. PMID: 19195433 Review. Spanish.
Cited by
-
Preexposure prophylaxis for HIV prevention: where have we been and where are we going?J Acquir Immune Defic Syndr. 2013 Jul;63 Suppl 2(0 2):S122-9. doi: 10.1097/QAI.0b013e3182986f69. J Acquir Immune Defic Syndr. 2013. PMID: 23764623 Free PMC article. Review.
-
Susceptibility to repeated, low-dose, rectal SHIVSF162P3 challenge is independent of TRIM5 genotype in rhesus macaques.AIDS Res Hum Retroviruses. 2013 Jul;29(7):1091-4. doi: 10.1089/aid.2012.0383. Epub 2013 Mar 29. AIDS Res Hum Retroviruses. 2013. PMID: 23461569 Free PMC article.
-
Protection Against Rectal Chimeric Simian/Human Immunodeficiency Virus Transmission in Macaques by Rectal-Specific Gel Formulations of Maraviroc and Tenofovir.J Infect Dis. 2015 Dec 15;212(12):1988-95. doi: 10.1093/infdis/jiv334. Epub 2015 Jun 12. J Infect Dis. 2015. PMID: 26071566 Free PMC article.
-
Global expression of molecular transporters in the human vaginal tract: implications for HIV chemoprophylaxis.PLoS One. 2013 Oct 15;8(10):e77340. doi: 10.1371/journal.pone.0077340. eCollection 2013. PLoS One. 2013. PMID: 24143220 Free PMC article.
-
Identification of Unequally Represented Founder Viruses Among Tissues in Very Early SIV Rectal Transmission.Front Microbiol. 2018 Mar 29;9:557. doi: 10.3389/fmicb.2018.00557. eCollection 2018. Front Microbiol. 2018. PMID: 29651274 Free PMC article.
References
-
- Agresti, A. 1996. An introduction to categorical data analysis. John Wiley & Sons, Inc., New York, NY.
-
- Balzarini, J., and L. Van Damme. 2007. Microbicide drug candidates to prevent HIV infection. Lancet 369:787-797. - PubMed
-
- Blakley, G. B., T. W. Beamer, and W. R. Dukelow. 1981. Characteristics of the menstrual cycle in nonhuman primates. IV. Timed mating in Macaca nemestrina. Lab. Anim. 15:351-353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

