Fundamental reaction mechanism and free energy profile for (-)-cocaine hydrolysis catalyzed by cocaine esterase
- PMID: 19642701
- PMCID: PMC2738781
- DOI: 10.1021/ja903990p
Fundamental reaction mechanism and free energy profile for (-)-cocaine hydrolysis catalyzed by cocaine esterase
Abstract
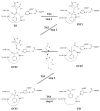
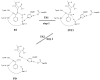
The fundamental reaction mechanism of cocaine esterase (CocE)-catalyzed hydrolysis of (-)-cocaine and the corresponding free energy profile have been studied by performing pseudobond first-principles quantum mechanical/molecular mechanical free energy (QM/MM-FE) calculations. On the basis of the QM/MM-FE results, the entire hydrolysis reaction consists of four reaction steps, including the nucleophilic attack on the carbonyl carbon of (-)-cocaine benzoyl ester by the hydroxyl group of Ser117, dissociation of (-)-cocaine benzoyl ester, nucleophilic attack on the carbonyl carbon of (-)-cocaine benzoyl ester by water, and finally dissociation between the (-)-cocaine benzoyl group and Ser117 of CocE. The third reaction step involving the nucleophilic attack of a water molecule was found to be rate-determining, which is remarkably different from (-)-cocaine hydrolysis catalyzed by wild-type butyrylcholinesterase (BChE; where the formation of the prereactive BChE-(-)-cocaine complex is rate-determining) or its mutants containing Tyr332Gly or Tyr332Ala mutation (where the first chemical reaction step is rate-determining). Besides, the role of Asp259 in the catalytic triad of CocE does not follow the general concept of the "charge-relay system" for all serine esterases. The free energy barrier calculated for the rate-determining step of CocE-catalyzed hydrolysis of (-)-cocaine is 17.9 kcal/mol, which is in good agreement with the experimentally derived activation free energy of 16.2 kcal/mol. In the present study, where many sodium ions are present, the effects of counterions are found to be significant in determining the free energy barrier. The finding of the significant effects of counterions on the free energy barrier may also be valuable in guiding future mechanistic studies on other charged enzymes.
Figures



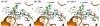
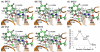
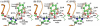

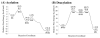
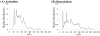
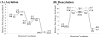
Similar articles
-
Reaction mechanism for cocaine esterase-catalyzed hydrolyses of (+)- and (-)-cocaine: unexpected common rate-determining step.J Phys Chem B. 2011 May 5;115(17):5017-25. doi: 10.1021/jp200975v. Epub 2011 Apr 12. J Phys Chem B. 2011. PMID: 21486046 Free PMC article.
-
Reaction Pathway and Free Energy Profile for Cocaine Hydrolase-Catalyzed Hydrolysis of (-)-Cocaine.J Chem Theory Comput. 2012 Apr 10;8(4):1426-1435. doi: 10.1021/ct200810d. Epub 2012 Mar 6. J Chem Theory Comput. 2012. PMID: 23066354 Free PMC article.
-
Reaction Pathway for Cocaine Hydrolase-Catalyzed Hydrolysis of (+)-Cocaine.Theor Chem Acc. 2016 Jan;135(1):15. doi: 10.1007/s00214-015-1788-2. Epub 2015 Dec 28. Theor Chem Acc. 2016. PMID: 28250715 Free PMC article.
-
The Rhodococcus sp. cocaine esterase: a bacterial candidate for novel pharmacokinetic-based therapies for cocaine abuse.IUBMB Life. 2003 Jul;55(7):397-402. doi: 10.1080/1521654031000138541. IUBMB Life. 2003. PMID: 14584590 Review.
-
Bacterial cocaine esterase: a protein-based therapy for cocaine overdose and addiction.Future Med Chem. 2012 Feb;4(2):137-50. doi: 10.4155/fmc.11.194. Future Med Chem. 2012. PMID: 22300094 Free PMC article. Review.
Cited by
-
Density functional theory investigation of cocaine water complexes.J Mol Model. 2013 Aug;19(8):3411-25. doi: 10.1007/s00894-013-1866-0. Epub 2013 May 18. J Mol Model. 2013. PMID: 23686284
-
Fundamental reaction pathway and free energy profile of proteasome inhibition by syringolin A (SylA).Org Biomol Chem. 2015 Jun 28;13(24):6857-65. doi: 10.1039/c5ob00737b. Org Biomol Chem. 2015. PMID: 26018983 Free PMC article.
-
Characterization of the structures of phosphodiesterase 10 binding with adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate by hybrid quantum mechanical/molecular mechanical calculations.J Phys Chem B. 2010 May 27;114(20):7022-8. doi: 10.1021/jp911527y. J Phys Chem B. 2010. PMID: 20443609 Free PMC article.
-
Fundamental reaction pathway and free energy profile for hydrolysis of intracellular second messenger adenosine 3',5'-cyclic monophosphate (cAMP) catalyzed by phosphodiesterase-4.J Phys Chem B. 2011 Oct 27;115(42):12208-19. doi: 10.1021/jp205509w. Epub 2011 Oct 5. J Phys Chem B. 2011. PMID: 21973014 Free PMC article.
-
Catalytic mechanism of cytochrome P450 for N-methylhydroxylation of nicotine: reaction pathways and regioselectivity of the enzymatic nicotine oxidation.Dalton Trans. 2013 Mar 21;42(11):3812-20. doi: 10.1039/c2dt32106h. Epub 2013 Jan 9. Dalton Trans. 2013. PMID: 23303461 Free PMC article.
References
-
- National Institute on Drug Abuse Cocaine Abuse and Addiction. (NIH Publication No. 99-4342).NIDA Research Report Series. 2004 (Rev. Nov. 2004)
-
- Substance Abuse and Mental Health Services Administration . Office of Applied Studies. Rockville, MD: 2006. Drug Abuse Warning Network, 2004: National Estimates of Drug-Related Emergency Department Visits. (DAWN Series D-28, DHHS Publication No. SMA 06-4143).
-
- Boelsterli UA, Goldlin C. Arch. Toxicol. 1991;65(5):351–60. - PubMed
-
- Hahn IH, Hoffman RS. Emerg. Med. Clin. N. Am. 2001;19(2):493–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

