TAF4/4b x TAF12 displays a unique mode of DNA binding and is required for core promoter function of a subset of genes
- PMID: 19635797
- PMCID: PMC2785316
- DOI: 10.1074/jbc.M109.011486
TAF4/4b x TAF12 displays a unique mode of DNA binding and is required for core promoter function of a subset of genes
Abstract
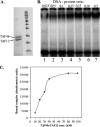
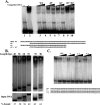
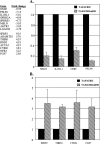
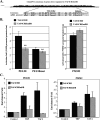
The major core promoter-binding factor in polymerase II transcription machinery is TFIID, a complex consisting of TBP, the TATA box-binding protein, and 13 to 14 TBP-associated factors (TAFs). Previously we found that the histone H2A-like TAF paralogs TAF4 and TAF4b possess DNA-binding activity. Whether TAF4/TAF4b DNA binding directs TFIID to a specific core promoter element or facilitates TFIID binding to established core promoter elements is not known. Here we analyzed the mode of TAF4b.TAF12 DNA binding and show that this complex binds DNA with high affinity. The DNA length required for optimal binding is approximately 70 bp. Although the complex displays a weak sequence preference, the nucleotide composition is less important than the length of the DNA for high affinity binding. Comparative expression profiling of wild-type and a DNA-binding mutant of TAF4 revealed common core promoter features in the down-regulated genes that include a TATA-box and an Initiator. Further examination of the PEL98 gene from this group showed diminished Initiator activity and TFIID occupancy in TAF4 DNA-binding mutant cells. These findings suggest that DNA binding by TAF4/4b-TAF12 facilitates the association of TFIID with the core promoter of a subset of genes.
Figures


Similar articles
-
TAF4 nucleates a core subcomplex of TFIID and mediates activated transcription from a TATA-less promoter.Proc Natl Acad Sci U S A. 2006 Aug 15;103(33):12347-52. doi: 10.1073/pnas.0605499103. Epub 2006 Aug 8. Proc Natl Acad Sci U S A. 2006. PMID: 16895980 Free PMC article.
-
A transcription factor IIA-binding site differentially regulates RNA polymerase II-mediated transcription in a promoter context-dependent manner.J Biol Chem. 2017 Jul 14;292(28):11873-11885. doi: 10.1074/jbc.M116.770412. Epub 2017 May 24. J Biol Chem. 2017. PMID: 28539359 Free PMC article.
-
TAF4, a subunit of transcription factor II D, directs promoter occupancy of nuclear receptor HNF4A during post-natal hepatocyte differentiation.Elife. 2014 Sep 10;3:e03613. doi: 10.7554/eLife.03613. Elife. 2014. PMID: 25209997 Free PMC article.
-
Developmental regulation of transcription initiation: more than just changing the actors.Curr Opin Genet Dev. 2010 Oct;20(5):533-40. doi: 10.1016/j.gde.2010.06.004. Epub 2010 Jul 2. Curr Opin Genet Dev. 2010. PMID: 20598874 Review.
-
Recent advances in understanding the structure and function of general transcription factor TFIID.Cell Mol Life Sci. 2009 Jul;66(13):2123-34. doi: 10.1007/s00018-009-0009-3. Epub 2009 Mar 24. Cell Mol Life Sci. 2009. PMID: 19308322 Free PMC article. Review.
Cited by
-
The H2A/H2B-like histone-fold domain proteins at the crossroad between chromatin and different DNA metabolisms.Transcription. 2013 May-Jun;4(3):114-9. doi: 10.4161/trns.25002. Epub 2013 May 16. Transcription. 2013. PMID: 23756340 Free PMC article.
-
Temporal association of herpes simplex virus ICP4 with cellular complexes functioning at multiple steps in PolII transcription.PLoS One. 2013 Oct 11;8(10):e78242. doi: 10.1371/journal.pone.0078242. eCollection 2013. PLoS One. 2013. PMID: 24147125 Free PMC article.
-
ZFP628 Is a TAF4b-Interacting Transcription Factor Required for Mouse Spermiogenesis.Mol Cell Biol. 2020 Mar 16;40(7):e00228-19. doi: 10.1128/MCB.00228-19. Print 2020 Mar 16. Mol Cell Biol. 2020. PMID: 31932482 Free PMC article.
-
αα-Hub domains and intrinsically disordered proteins: A decisive combo.J Biol Chem. 2021 Jan-Jun;296:100226. doi: 10.1074/jbc.REV120.012928. Epub 2020 Dec 29. J Biol Chem. 2021. PMID: 33361159 Free PMC article. Review.
-
Taenia solium TAF6 and TAF9 bind to a downstream promoter element present in the Tstbp1 gene core promoter.PLoS One. 2024 Aug 29;19(8):e0306633. doi: 10.1371/journal.pone.0306633. eCollection 2024. PLoS One. 2024. PMID: 39208271 Free PMC article.
References
-
- Juven-Gershon T., Hsu J. Y., Kadonaga J. T. (2006) Biochem. Soc. Trans. 34, 1047–1050 - PubMed
-
- Smale S. T. (2001) Genes Dev. 15, 2503–2508 - PubMed
-
- Smale S. T., Kadonaga J. T. (2003) Annu. Rev. Biochem. 72, 449–479 - PubMed
-
- Matangkasombut O., Auty R., Buratowski S. (2004) Adv. Protein Chem. 67, 67–92 - PubMed
-
- Sawadogo M., Roeder R. G. (1985) Cell 43, 165–175 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

