Mechanistic advances in plant natural product enzymes
- PMID: 19632140
- PMCID: PMC2749912
- DOI: 10.1016/j.cbpa.2009.06.019
Mechanistic advances in plant natural product enzymes
Abstract
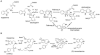
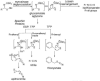
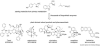
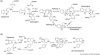
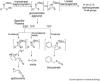
The biosynthetic pathways of plant natural products offer an abundance of knowledge to scientists in many fields. Synthetic chemists can be inspired by the synthetic strategies that nature uses to construct these compounds. Chemical and biological engineers are working to reprogram these biosynthetic pathways to more efficiently produce valuable products. Finally, biochemists and enzymologists are interested in the detailed mechanisms of the complex transformations involved in the construction of these natural products. Study of biosynthetic enzymes and pathways therefore has a wide-ranging impact. In recent years, many plant biosynthetic pathways have been characterized, particularly the pathways that are responsible for alkaloid biosynthesis. Here we highlight recently studied alkaloid biosynthetic enzymes that catalyze production of numerous complex medicinal compounds, as well as the specifier proteins in glucosinosolate biosynthesis, whose structure and mechanism of action are just beginning to be unraveled.
Figures

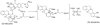
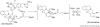
Similar articles
-
Recent progress in the metabolic engineering of alkaloids in plant systems.Curr Opin Biotechnol. 2013 Apr;24(2):354-65. doi: 10.1016/j.copbio.2012.08.003. Epub 2012 Sep 3. Curr Opin Biotechnol. 2013. PMID: 22954587 Free PMC article. Review.
-
Synthesis and trafficking of alkaloid biosynthetic enzymes.Curr Opin Plant Biol. 2005 Dec;8(6):657-66. doi: 10.1016/j.pbi.2005.09.008. Epub 2005 Sep 22. Curr Opin Plant Biol. 2005. PMID: 16182601 Review.
-
The Integration of Metabolomics and Next-Generation Sequencing Data to Elucidate the Pathways of Natural Product Metabolism in Medicinal Plants.Planta Med. 2018 Aug;84(12-13):855-873. doi: 10.1055/a-0630-1899. Epub 2018 May 29. Planta Med. 2018. PMID: 29843183 Review.
-
Tropane alkaloid biosynthesis: a centennial review.Nat Prod Rep. 2021 Sep 23;38(9):1634-1658. doi: 10.1039/d0np00076k. Nat Prod Rep. 2021. PMID: 33533391 Review.
-
Back to the plant: overcoming roadblocks to the microbial production of pharmaceutically important plant natural products.J Ind Microbiol Biotechnol. 2020 Oct;47(9-10):815-828. doi: 10.1007/s10295-020-02300-9. Epub 2020 Aug 9. J Ind Microbiol Biotechnol. 2020. PMID: 32772209 Review.
Cited by
-
Natural products as chemical probes.ACS Chem Biol. 2010 Jul 16;5(7):639-53. doi: 10.1021/cb100105c. ACS Chem Biol. 2010. PMID: 20509672 Free PMC article. Review.
References
-
- Dewick PM. Medicinal Natural Products: A Biosynthetic Approach. 2nd Ed. John Wiley & Sons, Inc.; 2004.
-
- Van der Heijden R, Jacobs DI, Snoeijer W, Didier H, Verpoorte R. The Catharanthus alkaloids: pharmacognosy and biotechnology. Curr Med Chem. 2004;11:607–628. - PubMed
-
- O’Connor SE, Maresh J. Chemistry and biology of monoterpene indole alkaloid biosynthesis. Nat Prod Rep. 2006;23:532–547. - PubMed
-
- DeLuca V, Laflamme PT. The Alkaloids: Chemistry and Biology. Academic Press; 1998.
-
- Wani M, Taylor H, Wall M, Coggon P, McPhail A. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971;93:2325–2327. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

