From carbohydrate leads to glycomimetic drugs
- PMID: 19629075
- PMCID: PMC7097102
- DOI: 10.1038/nrd2852
From carbohydrate leads to glycomimetic drugs
Abstract
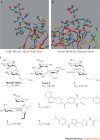
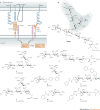
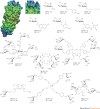
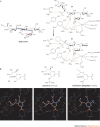
Carbohydrates are the most abundant natural products. Besides their role in metabolism and as structural building blocks, they are fundamental constituents of every cell surface, where they are involved in vital cellular recognition processes. Carbohydrates are a relatively untapped source of new drugs and therefore offer exciting new therapeutic opportunities. Advances in the functional understanding of carbohydrate-protein interactions have enabled the development of a new class of small-molecule drugs, known as glycomimetics. These compounds mimic the bioactive function of carbohydrates and address the drawbacks of carbohydrate leads, namely their low activity and insufficient drug-like properties. Here, we examine examples of approved carbohydrate-derived drugs, discuss the potential of carbohydrate-binding proteins as new drug targets (focusing on the lectin families) and consider ways to overcome the challenges of developing this unique class of novel therapeutics.
Conflict of interest statement
J.L.M. is an employee and shareholder of GlycoMimetics Inc.
Figures


Similar articles
-
Carbohydrate mimics and lectins: a source of new drugs and therapeutic opportunities.Mini Rev Med Chem. 2012 Dec;12(14):1434-42. doi: 10.2174/138955712803832690. Mini Rev Med Chem. 2012. PMID: 22827173 Review.
-
Biomimetic Carbohydrate-Binding Agents (CBAs): Binding Affinities and Biological Activities.Chembiochem. 2019 Jun 3;20(11):1329-1346. doi: 10.1002/cbic.201800742. Epub 2019 Apr 2. Chembiochem. 2019. PMID: 30644617 Review.
-
Glycomimetic drugs--a new source of therapeutic opportunities.Discov Med. 2009 Dec;8(43):247-52. Discov Med. 2009. PMID: 20040279
-
Novel approaches to glycomimetic design: development of small molecular weight lectin antagonists.Expert Opin Drug Discov. 2021 May;16(5):513-536. doi: 10.1080/17460441.2021.1857721. Epub 2021 Feb 5. Expert Opin Drug Discov. 2021. PMID: 33337918 Review.
-
Glycomimetics for the inhibition and modulation of lectins.Chem Soc Rev. 2023 Jun 6;52(11):3663-3740. doi: 10.1039/d2cs00954d. Chem Soc Rev. 2023. PMID: 37232696 Free PMC article. Review.
Cited by
-
Atomically resolved imaging of the conformations and adsorption geometries of individual β-cyclodextrins with non-contact AFM.Nat Commun. 2024 Nov 2;15(1):9482. doi: 10.1038/s41467-024-53555-0. Nat Commun. 2024. PMID: 39488518 Free PMC article.
-
Galectins in the Pathogenesis of Common Retinal Disease.Front Pharmacol. 2021 May 17;12:687495. doi: 10.3389/fphar.2021.687495. eCollection 2021. Front Pharmacol. 2021. PMID: 34079467 Free PMC article. Review.
-
Pentavalent Sialic Acid Conjugates Block Coxsackievirus A24 Variant and Human Adenovirus Type 37-Viruses That Cause Highly Contagious Eye Infections.ACS Chem Biol. 2020 Oct 16;15(10):2683-2691. doi: 10.1021/acschembio.0c00446. Epub 2020 Sep 16. ACS Chem Biol. 2020. PMID: 32845119 Free PMC article.
-
Identification of thioaptamer ligand against E-selectin: potential application for inflamed vasculature targeting.PLoS One. 2010 Sep 30;5(9):e13050. doi: 10.1371/journal.pone.0013050. PLoS One. 2010. PMID: 20927342 Free PMC article.
-
Pathoblockers or antivirulence drugs as a new option for the treatment of bacterial infections.Beilstein J Org Chem. 2018 Oct 11;14:2607-2617. doi: 10.3762/bjoc.14.239. eCollection 2018. Beilstein J Org Chem. 2018. PMID: 30410623 Free PMC article. Review.
References
-
- Van Teeffelen JW, Brands J, Stroes ES, Vink H. Endothelial glycocalyx: sweet shield of blood vessels. Trends Cardiovasc. Med. 2007;17:101–105. - PubMed
-
- Nieuwdorp M, et al. Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. J. Appl. Physiol. 2008;104:845–852. - PubMed
-
- Petitou M, et al. The synthetic pentasaccharide fondaparinux: first in the class of antithrombotic agents that selectively inhibit coagulation factor Xa. Semin. Thromb. Hemost. 2002;28:393–402. - PubMed
-
- Chen X, Zheng Y, Shen Y. Vogliobose (Basen, AO-128), one of the most important α-glucosidase inhibitors. Curr. Med. Chem. 2006;13:109–116. - PubMed
-
- Campbell LK, Baker DE, Campbell RK. Miglitol: assessment of its role in the treatment of patients with diabetes mellitus. Ann. Pharmacother. 2000;34:1291–1301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

