Distinct roles of FOXA2 and FOXA3 in allergic airway disease and asthma
- PMID: 19628779
- PMCID: PMC2753788
- DOI: 10.1164/rccm.200811-1768OC
Distinct roles of FOXA2 and FOXA3 in allergic airway disease and asthma
Abstract
Rationale: Increased production of mucus is a prominent feature of asthma. IL-13-driven mucous cell metaplasia is associated with decreased expression of the transcription factor FOXA2 and increased expression of the related transcription factor FOXA3 in animal and cell culture models.
Objectives: Establish how changes in FOXA2 and FOXA3 expression contribute to mucous metaplasia and determine whether FOXA2 and FOXA3 expression is altered in asthma.
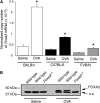
Methods: Mice expressing a Foxa2 transgene in airway epithelial cells and mice deficient in Foxa3 were analyzed after allergen sensitization and challenge. Expression of FOXA2, FOXA3, MUC5AC, and the highly IL-13-inducible gene CLCA1 was analyzed in airway biopsies from subjects with asthma and control subjects.
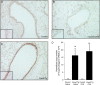
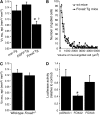
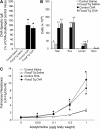
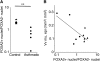
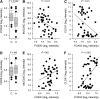
Measurements and main results: Expression of a Foxa2 transgene reduced allergen-induced mucous metaplasia by 45% compared with control transgenic mice (P < 0.05) whereas inactivation of Foxa3 had no detectable effects on mucous metaplasia. Expression of FOXA2 was reduced in subjects with asthma and was negatively correlated with MUC5AC and CLCA1 levels in subjects with asthma. In contrast, FOXA3 expression was not significantly correlated with MUC5AC and was positively correlated with CLCA1.
Conclusions: Increasing Foxa2 expression reduced mucous metaplasia in an allergic mouse model. Subjects with asthma had decreased FOXA2 expression, suggesting that therapeutic approaches that increase FOXA2 expression or function could be beneficial for reducing mucus production in asthma. Unlike FOXA2, FOXA3 did not regulate mucous metaplasia.
Figures
Similar articles
-
Foxa3 induces goblet cell metaplasia and inhibits innate antiviral immunity.Am J Respir Crit Care Med. 2014 Feb 1;189(3):301-13. doi: 10.1164/rccm.201306-1181OC. Am J Respir Crit Care Med. 2014. PMID: 24392884 Free PMC article.
-
Foxa2 regulates leukotrienes to inhibit Th2-mediated pulmonary inflammation.Am J Respir Cell Mol Biol. 2013 Dec;49(6):960-70. doi: 10.1165/rcmb.2013-0122OC. Am J Respir Cell Mol Biol. 2013. PMID: 23822876 Free PMC article.
-
Airway epithelial transcription factor NK2 homeobox 1 inhibits mucous cell metaplasia and Th2 inflammation.Am J Respir Crit Care Med. 2011 Aug 15;184(4):421-9. doi: 10.1164/rccm.201101-0106OC. Am J Respir Crit Care Med. 2011. PMID: 21562130 Free PMC article.
-
Inactivation of FOXA2 by Respiratory Bacterial Pathogens and Dysregulation of Pulmonary Mucus Homeostasis.Front Immunol. 2020 Mar 25;11:515. doi: 10.3389/fimmu.2020.00515. eCollection 2020. Front Immunol. 2020. PMID: 32269574 Free PMC article. Review.
-
Advances in mucous cell metaplasia: a plug for mucus as a therapeutic focus in chronic airway disease.Am J Respir Cell Mol Biol. 2010 Mar;42(3):268-75. doi: 10.1165/rcmb.2009-0151TR. Epub 2009 Jun 11. Am J Respir Cell Mol Biol. 2010. PMID: 19520914 Free PMC article. Review.
Cited by
-
Epithelial miR-141 regulates IL-13-induced airway mucus production.JCI Insight. 2021 Mar 8;6(5):e139019. doi: 10.1172/jci.insight.139019. JCI Insight. 2021. PMID: 33682796 Free PMC article.
-
Genes associated with MUC5AC expression in small airway epithelium of human smokers and non-smokers.BMC Med Genomics. 2012 Jun 7;5:21. doi: 10.1186/1755-8794-5-21. BMC Med Genomics. 2012. PMID: 22676183 Free PMC article.
-
Molecular characterization of pulmonary defenses against bacterial invasion in allergic asthma: The role of Foxa2 in regulation of β-defensin 1.PLoS One. 2019 Dec 27;14(12):e0226517. doi: 10.1371/journal.pone.0226517. eCollection 2019. PLoS One. 2019. PMID: 31881038 Free PMC article.
-
Forkhead box protein A2 and T helper type 2-mediated pulmonary inflammation.World J Methodol. 2015 Dec 26;5(4):223-9. doi: 10.5662/wjm.v5.i4.223. eCollection 2015 Dec 26. World J Methodol. 2015. PMID: 26713283 Free PMC article. Review.
-
Single-cell RNA-sequencing in asthma research.Front Immunol. 2022 Nov 29;13:988573. doi: 10.3389/fimmu.2022.988573. eCollection 2022. Front Immunol. 2022. PMID: 36524132 Free PMC article. Review.
References
-
- Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest 1992;101:916–921. - PubMed
-
- Ordonez CL, Khashayar R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, Zhang Y, Novikov A, Dolganov G, et al. Mild and moderate asthma is associated with airway goblet cell hyperplasia and abnormalities in mucin gene expression. Am J Respir Crit Care Med 2001;163:517–523. - PubMed
-
- Rogers DF. Airway mucus hypersecretion in asthma: an undervalued pathology? Curr Opin Pharmacol 2004;4:241–250. - PubMed
-
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev 2006;86:245–278. - PubMed
-
- Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol 2006;34:661–665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

