Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission
- PMID: 19587241
- PMCID: PMC2715537
- DOI: 10.1073/pnas.0901226106
Interactions between mutualist Wigglesworthia and tsetse peptidoglycan recognition protein (PGRP-LB) influence trypanosome transmission
Abstract
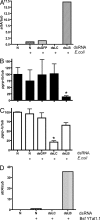
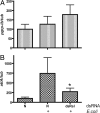
Tsetse flies, the sole vectors of African trypanosomes, have coevolved with mutualistic endosymbiont Wigglesworthia glossinidiae. Elimination of Wigglesworthia renders tsetse sterile and increases their trypanosome infection susceptibility. We show that a tsetse peptidoglycan recognition protein (PGRP-LB) is crucial for symbiotic tolerance and trypanosome infection processes. Tsetse pgrp-lb is expressed in the Wigglesworthia-harboring organ (bacteriome) in the midgut, and its level of expression correlates with symbiont numbers. Adult tsetse cured of Wigglesworthia infections have significantly lower pgrp-lb levels than corresponding normal adults. RNA interference (RNAi)-mediated depletion of pgrp-lb results in the activation of the immune deficiency (IMD) signaling pathway and leads to the synthesis of antimicrobial peptides (AMPs), which decrease Wigglesworthia density. Depletion of pgrp-lb also increases the host's susceptibility to trypanosome infections. Finally, parasitized adults have significantly lower pgrp-lb levels than flies, which have successfully eliminated trypanosome infections. When both PGRP-LB and IMD immunity pathway functions are blocked, flies become unusually susceptible to parasitism. Based on the presence of conserved amidase domains, tsetse PGRP-LB may scavenge the peptidoglycan (PGN) released by Wigglesworthia and prevent the activation of symbiont-damaging host immune responses. In addition, tsetse PGRP-LB may have an anti-protozoal activity that confers parasite resistance. The symbiotic adaptations and the limited exposure of tsetse to foreign microbes may have led to the considerable differences in pgrp-lb expression and regulation noted in tsetse from that of closely related Drosophila. A dynamic interplay between Wigglesworthia and host immunity apparently is influential in tsetse's ability to transmit trypanosomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
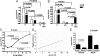
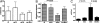
Similar articles
-
PGRP-LB is a maternally transmitted immune milk protein that influences symbiosis and parasitism in tsetse's offspring.Proc Natl Acad Sci U S A. 2012 Jun 26;109(26):10552-7. doi: 10.1073/pnas.1116431109. Epub 2012 Jun 11. Proc Natl Acad Sci U S A. 2012. PMID: 22689989 Free PMC article.
-
Mutualist-Provisioned Resources Impact Vector Competency.mBio. 2019 Jun 4;10(3):e00018-19. doi: 10.1128/mBio.00018-19. mBio. 2019. PMID: 31164458 Free PMC article.
-
Unravelling the relationship between the tsetse fly and its obligate symbiont Wigglesworthia: transcriptomic and metabolomic landscapes reveal highly integrated physiological networks.Proc Biol Sci. 2017 Jun 28;284(1857):20170360. doi: 10.1098/rspb.2017.0360. Proc Biol Sci. 2017. PMID: 28659447 Free PMC article.
-
Bacterial Symbionts of Tsetse Flies: Relationships and Functional Interactions Between Tsetse Flies and Their Symbionts.Results Probl Cell Differ. 2020;69:497-536. doi: 10.1007/978-3-030-51849-3_19. Results Probl Cell Differ. 2020. PMID: 33263885 Review.
-
What can a weevil teach a fly, and reciprocally? Interaction of host immune systems with endosymbionts in Glossina and Sitophilus.BMC Microbiol. 2018 Nov 23;18(Suppl 1):150. doi: 10.1186/s12866-018-1278-5. BMC Microbiol. 2018. PMID: 30470176 Free PMC article. Review.
Cited by
-
Obligate symbionts activate immune system development in the tsetse fly.J Immunol. 2012 Apr 1;188(7):3395-403. doi: 10.4049/jimmunol.1103691. Epub 2012 Feb 24. J Immunol. 2012. PMID: 22368278 Free PMC article.
-
Trypanosome Transmission Dynamics in Tsetse.Curr Opin Insect Sci. 2014 Sep;3:43-49. doi: 10.1016/j.cois.2014.07.003. Curr Opin Insect Sci. 2014. PMID: 25580379 Free PMC article.
-
OmpA-mediated biofilm formation is essential for the commensal bacterium Sodalis glossinidius to colonize the tsetse fly gut.Appl Environ Microbiol. 2012 Nov;78(21):7760-8. doi: 10.1128/AEM.01858-12. Epub 2012 Aug 31. Appl Environ Microbiol. 2012. PMID: 22941073 Free PMC article.
-
Interactions between Leishmania parasite and sandfly: a review.Parasitol Res. 2023 Dec 6;123(1):6. doi: 10.1007/s00436-023-08043-7. Parasitol Res. 2023. PMID: 38052752 Review.
-
Tsetse peritrophic matrix influences for trypanosome transmission.J Insect Physiol. 2019 Oct;118:103919. doi: 10.1016/j.jinsphys.2019.103919. Epub 2019 Aug 16. J Insect Physiol. 2019. PMID: 31425686 Free PMC article. Review.
References
-
- Dale C, Welburn SC. The endosymbionts of tsetse flies: Manipulating host-parasite interactions. Int J Parisitol. 2001;31:628–631. - PubMed
-
- Aksoy S. Wigglesworthia gen. nov. and Wigglesworthia glossinidia sp. nov., taxa consisting of the mycetocyte-associated, primary endosymbionts of tsetse flies. Int J Syst. Bacteriol. 1995;45:848–851. - PubMed
-
- Cheng Q, Aksoy S. Tissue tropism, transmission, and expression of foreign genes in vivo in midgut symbionts of tsetse flies. Insect Mol Biol. 1999;8:125–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

