EGFRvIII and c-Met pathway inhibitors synergize against PTEN-null/EGFRvIII+ glioblastoma xenografts
- PMID: 19584231
- PMCID: PMC2846399
- DOI: 10.1158/1535-7163.MCT-09-0188
EGFRvIII and c-Met pathway inhibitors synergize against PTEN-null/EGFRvIII+ glioblastoma xenografts
Abstract
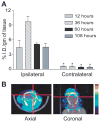
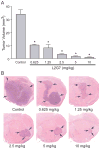
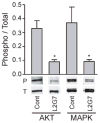
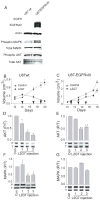
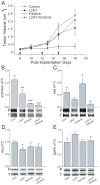
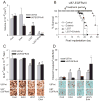
Receptor tyrosine kinase (RTK) systems, such as hepatocyte growth factor (HGF) and its receptor c-Met, and epidermal growth factor receptor (EGFR), are responsible for the malignant progression of multiple solid tumors. Recent research shows that these RTK systems comodulate overlapping and dynamically adaptable oncogenic downstream signaling pathways. This study investigates how EGFRvIII, a constitutively active EGFR deletion mutant, alters tumor growth and signaling responses to RTK inhibition in PTEN-null/HGF(+)/c-Met(+) glioma xenografts. We show that a neutralizing anti-HGF monoclonal antibody (L2G7) potently inhibits tumor growth and the activation of Akt and mitogen-activated protein kinase (MAPK) in PTEN-null/HGF(+)/c-Met(+)/EGFRvIII(-) U87 glioma xenografts (U87wt). Isogenic EGFRvIII(+) U87 xenografts (U87-EGFRvIII), which grew five times more rapidly than U87-wt xenografts, were unresponsive to EGFRvIII inhibition by erlotinib and were only minimally responsive to anti-HGF monoclonal antibodies. EGFRvIII expression diminished the magnitude of Akt inhibition and completely prevented MAPK inhibition by L2G7. Despite the lack of response to L2G7 or erlotinib as single agents, their combination synergized to produce substantial antitumor effects (inhibited tumor cell proliferation, enhanced apoptosis, arrested tumor growth, prolonged animal survival), against subcutaneous and orthotopic U87-EGFRvIII xenografts. The dramatic response to combining HGF:c-Met and EGFRvIII pathway inhibitors in U87-EGFRvIII xenografts occurred in the absence of Akt and MAPK inhibition. These findings show that combining c-Met and EGFRvIII pathway inhibitors can generate potent antitumor effects in PTEN-null tumors. They also provide insights into how EGFRvIII and c-Met may alter signaling networks and reveal the potential limitations of certain biochemical biomarkers to predict the efficacy of RTK inhibition in genetically diverse cancers.
Figures
Similar articles
-
Crizotinib and erlotinib inhibits growth of c-Met+/EGFRvIII+ primary human glioblastoma xenografts.Clin Neurol Neurosurg. 2018 Aug;171:26-33. doi: 10.1016/j.clineuro.2018.02.041. Epub 2018 Mar 9. Clin Neurol Neurosurg. 2018. PMID: 29803091
-
PTEN reconstitution alters glioma responses to c-Met pathway inhibition.Anticancer Drugs. 2011 Oct;22(9):905-12. doi: 10.1097/CAD.0b013e3283484750. Anticancer Drugs. 2011. PMID: 21654317 Free PMC article.
-
The plasticity of oncogene addiction: implications for targeted therapies directed to receptor tyrosine kinases.Neoplasia. 2009 May;11(5):448-58, 2 p following 458. doi: 10.1593/neo.09230. Neoplasia. 2009. PMID: 19412429 Free PMC article.
-
The Role of EGFR-Met Interactions in the Pathogenesis of Glioblastoma and Resistance to Treatment.Curr Cancer Drug Targets. 2017;17(3):297-302. doi: 10.2174/1568009616666161215162515. Curr Cancer Drug Targets. 2017. PMID: 28004613 Review.
-
EGFR-targeted therapy in malignant glioma: novel aspects and mechanisms of drug resistance.Curr Mol Pharmacol. 2010 Jan;3(1):37-52. doi: 10.2174/1874467211003010037. Curr Mol Pharmacol. 2010. PMID: 20030624 Free PMC article. Review.
Cited by
-
Emerging antibody combinations in oncology.MAbs. 2011 Jul-Aug;3(4):338-51. doi: 10.4161/mabs.3.4.16615. Epub 2011 Jul 1. MAbs. 2011. PMID: 21697653 Free PMC article. Review.
-
Targeting the epithelial to mesenchymal transition in glioblastoma: the emerging role of MET signaling.Onco Targets Ther. 2014 Oct 20;7:1933-44. doi: 10.2147/OTT.S36582. eCollection 2014. Onco Targets Ther. 2014. PMID: 25364264 Free PMC article. Review.
-
Genomic profiling of a Hepatocyte growth factor-dependent signature for MET-targeted therapy in glioblastoma.J Transl Med. 2015 Sep 17;13:306. doi: 10.1186/s12967-015-0667-x. J Transl Med. 2015. PMID: 26381735 Free PMC article.
-
Gefitinib induces apoptosis in human glioma cells by targeting Bad phosphorylation.J Neurooncol. 2011 Dec;105(3):507-22. doi: 10.1007/s11060-011-0632-3. Epub 2011 Jul 9. J Neurooncol. 2011. PMID: 21744078
-
Multivariate signaling regulation by SHP2 differentially controls proliferation and therapeutic response in glioma cells.J Cell Sci. 2014 Aug 15;127(Pt 16):3555-67. doi: 10.1242/jcs.150862. Epub 2014 Jun 20. J Cell Sci. 2014. PMID: 24951116 Free PMC article.
References
-
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316(5827):1039–43. - PubMed
-
- Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318(5848):287–90. - PubMed
-
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4(12):915–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

