Cadherin and integrin regulation of epithelial cell migration
- PMID: 19583181
- PMCID: PMC3556267
- DOI: 10.1021/la901109e
Cadherin and integrin regulation of epithelial cell migration
Abstract
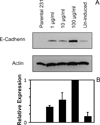
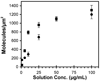
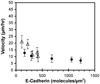
These studies quantified the relative effects of E-cadherin expression and homophilic ligation on the integrin-mediated motility of epithelial cells. Micropatterned proteins were used to quantitatively titrate the ligation of E-cadherin and integrin receptors in order to assess their coordinate influence on the migration velocities of MDA-MB-231 breast tumor epithelial cells. Fibronectin, E-cadherin, and mixtures of fibronectin and E-cadherin were covalently patterned on solid surfaces at defined compositions and mass coverages. The migration velocities of parental epithelial cells and of cells engineered to express E-cadherin under tetracycline control show that E-cadherin expression reduces cell motility by both adhesion-dependent and adhesion-independent mechanisms. Increasing E-cadherin expression levels also suppresses the dependence of cell velocity on the fibronectin coverage. On E-cadherin-containing substrata, the cell velocity decreases both with the E-cadherin expression level and with the immobilized E-cadherin surface density. These studies thus identified conditions under which E-cadherin preferentially suppresses cell migration by adhesion-independent versus adhesion-dependent mechanisms.
Figures



Similar articles
-
Regulation of cell motile behavior by crosstalk between cadherin- and integrin-mediated adhesions.Proc Natl Acad Sci U S A. 2010 Jul 27;107(30):13324-9. doi: 10.1073/pnas.1002662107. Epub 2010 Jun 21. Proc Natl Acad Sci U S A. 2010. PMID: 20566866 Free PMC article.
-
Molecular basis for leukocyte integrin alpha(E)beta(7) adhesion to epithelial (E)-cadherin.J Exp Med. 2000 May 1;191(9):1555-67. doi: 10.1084/jem.191.9.1555. J Exp Med. 2000. PMID: 10790430 Free PMC article.
-
Integrin alpha3beta1 engagement disrupts intercellular adhesion.Exp Cell Res. 2001 Jan 15;262(2):180-96. doi: 10.1006/excr.2000.5083. Exp Cell Res. 2001. PMID: 11139342
-
The SRC-induced mesenchymal state in late-stage colon cancer cells.Cells Tissues Organs. 2005;179(1-2):73-80. doi: 10.1159/000084511. Cells Tissues Organs. 2005. PMID: 15942195 Review.
-
Cadherin profiling for therapeutic interventions in Epithelial Mesenchymal Transition (EMT) and tumorigenesis.Exp Cell Res. 2018 Jul 15;368(2):137-146. doi: 10.1016/j.yexcr.2018.04.014. Epub 2018 Apr 16. Exp Cell Res. 2018. PMID: 29674112 Review.
Cited by
-
Innovative method for quantification of cell-cell adhesion in 96-well plates.Cell Adh Migr. 2011 May-Jun;5(3):215-9. doi: 10.4161/cam.5.3.14648. Epub 2011 May 1. Cell Adh Migr. 2011. PMID: 21339704 Free PMC article.
-
Mucosal healing and inflammatory bowel disease: Therapeutic implications and new targets.World J Gastroenterol. 2023 Feb 21;29(7):1157-1172. doi: 10.3748/wjg.v29.i7.1157. World J Gastroenterol. 2023. PMID: 36926666 Free PMC article. Review.
-
The Interaction of Mechanics and the Hippo Pathway in Drosophila melanogaster.Cancers (Basel). 2023 Oct 3;15(19):4840. doi: 10.3390/cancers15194840. Cancers (Basel). 2023. PMID: 37835534 Free PMC article.
-
Thymosin β4 Regulates the Differentiation of Thymocytes by Controlling the Cytoskeletal Rearrangement and Mitochondrial Transfer of Thymus Epithelial Cells.Int J Mol Sci. 2024 Jan 16;25(2):1088. doi: 10.3390/ijms25021088. Int J Mol Sci. 2024. PMID: 38256161 Free PMC article.
-
Astrocytes in Migration.Neurochem Res. 2017 Jan;42(1):272-282. doi: 10.1007/s11064-016-2089-4. Epub 2016 Nov 11. Neurochem Res. 2017. PMID: 27837318 Review.
References
-
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4(2):118–132. - PubMed
-
- Janda E, Nevolo M, Lehmann K, Downward J, Beug H, Grieco M. Raf plus TGFbeta-dependent EMT is initiated by endocytosis and lysosomal degradation of E-cadherin. Oncogene. 2006;25(54):7117–7130. - PubMed
-
- Hazan RB, Qiao R, Keren R, Badano I, Suyama K. Cadherin switch in tumor progression. Ann N Y Acad Sci. 2004;1014:155–163. - PubMed
-
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5(5):806–811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

