Mechanisms of growth and homeostasis in the Drosophila wing
- PMID: 19575645
- PMCID: PMC2760035
- DOI: 10.1146/annurev.cellbio.24.110707.175242
Mechanisms of growth and homeostasis in the Drosophila wing
Abstract
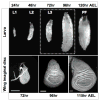
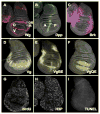
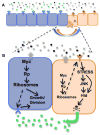
Animal shape and size is controlled with amazing precision during development. External factors such as nutrient availability and crowding can alter overall animal size, but individual body parts scale reproducibly to match the body even with challenges from a changing environment. How is such precision achieved? Here, we review selected research from the last few years in Drosophila--arguably the premier genetic model for the study of animal growth--that sheds light on how body and tissue size are regulated by forces intrinsic to individual organs. We focus on two topics currently under intense study: the influence of pattern regulators on organ and tissue growth and the role of local competitive interactions between cells in tissue homeostasis and final size.
Figures

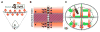

Similar articles
-
The wing and the eye: a parsimonious theory for scaling and growth control?Wiley Interdiscip Rev Dev Biol. 2015 Nov-Dec;4(6):591-608. doi: 10.1002/wdev.195. Epub 2015 Jun 24. Wiley Interdiscip Rev Dev Biol. 2015. PMID: 26108346 Review.
-
Growth and cell survival are unevenly impaired in pixie mutant wing discs.Development. 2005 Dec;132(24):5411-24. doi: 10.1242/dev.02148. Epub 2005 Nov 16. Development. 2005. PMID: 16291791
-
Anterior-posterior patterning of Drosophila wing discs I: A baseline mathematical model.Math Biosci. 2019 Aug;314:13-27. doi: 10.1016/j.mbs.2019.05.001. Epub 2019 May 11. Math Biosci. 2019. PMID: 31085191
-
Notch signalling coordinates tissue growth and wing fate specification in Drosophila.Development. 2008 Dec;135(24):3995-4001. doi: 10.1242/dev.027789. Epub 2008 Nov 5. Development. 2008. PMID: 18987026
-
Forces controlling organ growth and size.Mech Dev. 2017 Apr;144(Pt A):53-61. doi: 10.1016/j.mod.2016.11.005. Epub 2016 Nov 30. Mech Dev. 2017. PMID: 27913118 Review.
Cited by
-
Asymmetric flies: the control of developmental noise in Drosophila.Fly (Austin). 2013 Apr-Jun;7(2):70-7. doi: 10.4161/fly.23558. Epub 2013 Mar 21. Fly (Austin). 2013. PMID: 23519089 Free PMC article.
-
Transcriptome profiling for developmental stages Protaetia brevitarsis seulensis with focus on wing development and metamorphosis.PLoS One. 2023 Mar 1;18(3):e0277815. doi: 10.1371/journal.pone.0277815. eCollection 2023. PLoS One. 2023. PMID: 36857331 Free PMC article.
-
A docked mutation phenocopies dumpy oblique alleles via altered vesicle trafficking.PeerJ. 2021 Oct 13;9:e12175. doi: 10.7717/peerj.12175. eCollection 2021. PeerJ. 2021. PMID: 34721959 Free PMC article.
-
Xrn1/Pacman affects apoptosis and regulates expression of hid and reaper.Biol Open. 2015 Apr 2;4(5):649-60. doi: 10.1242/bio.201410199. Biol Open. 2015. PMID: 25836675 Free PMC article.
-
Causes of variability in estimates of mutational variance from mutation accumulation experiments.Genetics. 2022 May 31;221(2):iyac060. doi: 10.1093/genetics/iyac060. Genetics. 2022. PMID: 35435211 Free PMC article.
References
-
- Adachi-Yamada T, Fujimura-Kamada K, Nishida Y, Matsumoto K. Distortion of proximodistal information causxes JNK-dependent apoptosis in Drosophila wing. Nature. 1999;400:166–9. - PubMed
-
- Adachi-Yamada T, O’Connor MB. Morphogenetic apoptosis: a mechanism for correcting discontinuities in morphogen gradients. Dev Biol. 2002;251:74–90. - PubMed
-
- Affolter M, Basler K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet. 2007;8:663–74. - PubMed
-
- Basler K, Struhl G. Compartment boundaries and the control of Drosophila limb pattern by hedgehog protein. Cell. 1994;76:89–102. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

