Mature adult dystrophic mouse muscle environment does not impede efficient engrafted satellite cell regeneration and self-renewal
- PMID: 19575422
- PMCID: PMC3836226
- DOI: 10.1002/stem.162
Mature adult dystrophic mouse muscle environment does not impede efficient engrafted satellite cell regeneration and self-renewal
Abstract
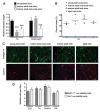
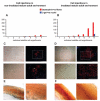
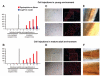
Changes that occur in the skeletal muscle environment with the progress of muscular dystrophies may affect stem cell function and result in impaired muscle regeneration. It has previously been suggested that the success of stem cell transplantation could therefore be dependent both on the properties of the cell itself and on the host muscle environment. Here we engrafted young and mature adult mdx-nude mice, which are the genetic homolog of Duchenne muscular dystrophy, with a small number of satellite cells freshly isolated from young, normal donor mice. We found that the donor satellite cells contributed to muscle regeneration and self-renewal as efficiently within mature adult, as in young, dystrophic host muscle. Donor-derived satellite cells also contributed to robust regeneration after further injury, showing that they were functional despite the more advanced dystrophic muscle environment. These findings provide evidence that muscle tissue in a later stage of dystrophy may be effectively treated by stem cells.
Figures

Similar articles
-
Donor satellite cell engraftment is significantly augmented when the host niche is preserved and endogenous satellite cells are incapacitated.Stem Cells. 2012 Sep;30(9):1971-84. doi: 10.1002/stem.1158. Stem Cells. 2012. PMID: 22730231 Free PMC article.
-
Isolation, culture, and transplantation of muscle satellite cells.J Vis Exp. 2014 Apr 8;(86):50846. doi: 10.3791/50846. J Vis Exp. 2014. PMID: 24747722 Free PMC article.
-
Satellite cells from dystrophic muscle retain regenerative capacity.Stem Cell Res. 2015 Jan;14(1):20-9. doi: 10.1016/j.scr.2014.10.007. Epub 2014 Nov 1. Stem Cell Res. 2015. PMID: 25460248 Free PMC article.
-
Recent progress in satellite cell/myoblast engraftment -- relevance for therapy.FEBS J. 2013 Sep;280(17):4281-93. doi: 10.1111/febs.12273. Epub 2013 Apr 24. FEBS J. 2013. PMID: 23560812 Free PMC article. Review.
-
Satellite Cells in Muscular Dystrophy - Lost in Polarity.Trends Mol Med. 2016 Jun;22(6):479-496. doi: 10.1016/j.molmed.2016.04.002. Epub 2016 May 5. Trends Mol Med. 2016. PMID: 27161598 Free PMC article. Review.
Cited by
-
The satellite cell in male and female, developing and adult mouse muscle: distinct stem cells for growth and regeneration.PLoS One. 2012;7(5):e37950. doi: 10.1371/journal.pone.0037950. Epub 2012 May 25. PLoS One. 2012. PMID: 22662253 Free PMC article.
-
Are human and mouse satellite cells really the same?J Histochem Cytochem. 2010 Nov;58(11):941-55. doi: 10.1369/jhc.2010.956201. Epub 2010 Jul 19. J Histochem Cytochem. 2010. PMID: 20644208 Free PMC article. Review.
-
Grafting of a single donor myofibre promotes hypertrophy in dystrophic mouse muscle.PLoS One. 2013;8(1):e54599. doi: 10.1371/journal.pone.0054599. Epub 2013 Jan 18. PLoS One. 2013. PMID: 23349935 Free PMC article.
-
Assessment of the myogenic stem cell compartment following transplantation of Pax3/Pax7-induced embryonic stem cell-derived progenitors.Stem Cells. 2011 May;29(5):777-90. doi: 10.1002/stem.625. Stem Cells. 2011. PMID: 21374762 Free PMC article.
-
Impaired Regeneration in Dystrophic Muscle-New Target for Therapy.Front Mol Neurosci. 2020 May 25;13:69. doi: 10.3389/fnmol.2020.00069. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32523512 Free PMC article.
References
-
- Gopinath SD, Rando TA. Stem cell review series: aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7:590–598. - PubMed
-
- Decary S, Hamida CB, Mouly V, et al. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord. 2000;10:113–120. - PubMed
-
- Collins CA, Olsen I, Zammit PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. - PubMed
-
- Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256:C1262–C1266. - PubMed

