Protective and pathologic roles of the immune response to mouse hepatitis virus type 1: implications for severe acute respiratory syndrome
- PMID: 19570864
- PMCID: PMC2738266
- DOI: 10.1128/JVI.00355-09
Protective and pathologic roles of the immune response to mouse hepatitis virus type 1: implications for severe acute respiratory syndrome
Abstract
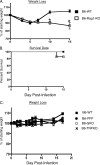
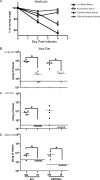
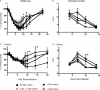
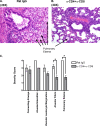
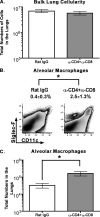
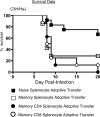
Intranasal mouse hepatitis virus type 1 (MHV-1) infection of mice induces lung pathology similar to that observed in severe acute respiratory syndrome (SARS) patients. However, the severity of MHV-1-induced pulmonary disease varies among mouse strains, and it has been suggested that differences in the host immune response might account for this variation. It has also been suggested that immunopathology may represent an important clinical feature of SARS. Little is known about the host immune response to MHV-1 and how it might contribute to some of the pathological changes detected in infected mice. In this study we show that an intact type I interferon system and the adaptive immune responses are required for controlling MHV-1 replication and preventing morbidity and mortality in resistant C57BL/6J mice after infection. The NK cell response also helps minimize the severity of illness following MHV-1 infection of C57BL/6J mice. In A/J and C3H/HeJ mice, which are highly susceptible to MHV-1-induced disease, we demonstrate that both CD4 and CD8 T cells contribute to morbidity during primary infection, and memory responses can enhance morbidity and mortality during subsequent reexposure to MHV-1. However, morbidity in A/J and C3H/HeJ mice can be minimized by treating them with immune serum prior to MHV-1 infection. Overall, our findings highlight the role of the host immune response in contributing to the pathogenesis of coronavirus-induced respiratory disease.
Figures


Similar articles
-
Toll-like receptor 4 deficiency increases disease and mortality after mouse hepatitis virus type 1 infection of susceptible C3H mice.J Virol. 2009 Sep;83(17):8946-56. doi: 10.1128/JVI.01857-08. Epub 2009 Jun 24. J Virol. 2009. PMID: 19553337 Free PMC article.
-
Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice.J Virol. 2006 Nov;80(21):10382-94. doi: 10.1128/JVI.00747-06. J Virol. 2006. PMID: 17041219 Free PMC article.
-
Depletion of alveolar macrophages ameliorates virus-induced disease following a pulmonary coronavirus infection.PLoS One. 2014 Mar 7;9(3):e90720. doi: 10.1371/journal.pone.0090720. eCollection 2014. PLoS One. 2014. PMID: 24608125 Free PMC article.
-
Pathogenesis of acute and chronic central nervous system infection with variants of mouse hepatitis virus, strain JHM.Immunol Res. 2007;39(1-3):160-72. doi: 10.1007/s12026-007-0079-y. Immunol Res. 2007. PMID: 17917063 Free PMC article. Review.
-
MHV infection of the CNS: mechanisms of immune-mediated control.Viral Immunol. 2001;14(1):1-18. doi: 10.1089/08828240151061329. Viral Immunol. 2001. PMID: 11270593 Review.
Cited by
-
Use of compressed sensing to expedite high-throughput diagnostic testing for COVID-19 and beyond.PLoS Comput Biol. 2022 Oct 24;18(10):e1010629. doi: 10.1371/journal.pcbi.1010629. eCollection 2022 Oct. PLoS Comput Biol. 2022. PMID: 36279287 Free PMC article.
-
Defining Parameters That Modulate Susceptibility and Protection to Respiratory Murine Coronavirus MHV1 Infection.J Immunol. 2024 Feb 15;212(4):563-575. doi: 10.4049/jimmunol.2300434. J Immunol. 2024. PMID: 38149923
-
Priming With Rhinovirus Protects Mice Against a Lethal Pulmonary Coronavirus Infection.Front Immunol. 2022 May 30;13:886611. doi: 10.3389/fimmu.2022.886611. eCollection 2022. Front Immunol. 2022. PMID: 35711419 Free PMC article.
-
WFDC1/ps20: a host factor that influences the neutrophil response to murine hepatitis virus (MHV) 1 infection.Antiviral Res. 2012 Nov;96(2):158-68. doi: 10.1016/j.antiviral.2012.08.012. Epub 2012 Sep 6. Antiviral Res. 2012. PMID: 22960155 Free PMC article.
-
Virus-Specific Regulatory T Cells Persist as Memory in a Neurotropic Coronavirus Infection.J Immunol. 2022 Apr 15;208(8):1989-1997. doi: 10.4049/jimmunol.2100794. Epub 2022 Apr 1. J Immunol. 2022. PMID: 35365567 Free PMC article.
References
-
- Badovinac, V. P., S. E. Hamilton, and J. T. Harty. 2003. Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity 18:463-474. - PubMed
-
- Badovinac, V. P., and J. T. Harty. 2006. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol. Rev. 211:67-80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

