Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression
- PMID: 19570822
- PMCID: PMC2824855
- DOI: 10.4049/jimmunol.0901363
Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression
Abstract
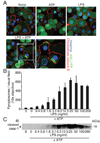
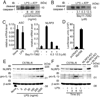
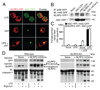
The IL-1 family cytokines are regulated on transcriptional and posttranscriptional levels. Pattern recognition and cytokine receptors control pro-IL-1beta transcription whereas inflammasomes regulate the proteolytic processing of pro-IL-1beta. The NLRP3 inflammasome, however, assembles in response to extracellular ATP, pore-forming toxins, or crystals only in the presence of proinflammatory stimuli. How the activation of gene transcription by signaling receptors enables NLRP3 activation remains elusive and controversial. In this study, we show that cell priming through multiple signaling receptors induces NLRP3 expression, which we identified to be a critical checkpoint for NLRP3 activation. Signals provided by NF-kappaB activators are necessary but not sufficient for NLRP3 activation, and a second stimulus such as ATP or crystal-induced damage is required for NLRP3 activation.
Figures

Similar articles
-
Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages.Mol Immunol. 2013 Dec;56(4):471-9. doi: 10.1016/j.molimm.2013.05.005. Epub 2013 Aug 1. Mol Immunol. 2013. PMID: 23911403
-
Fucoxanthin from microalgae Phaeodactylum tricornutum inhibits pro-inflammatory cytokines by regulating both NF-κB and NLRP3 inflammasome activation.Sci Rep. 2021 Jan 12;11(1):543. doi: 10.1038/s41598-020-80748-6. Sci Rep. 2021. PMID: 33436909 Free PMC article.
-
Activation of the Nlrp3 inflammasome by Streptococcus pyogenes requires streptolysin O and NF-kappa B activation but proceeds independently of TLR signaling and P2X7 receptor.J Immunol. 2009 Nov 1;183(9):5823-9. doi: 10.4049/jimmunol.0900444. Epub 2009 Oct 7. J Immunol. 2009. PMID: 19812205 Free PMC article.
-
Pathogenic Vibrio activate NLRP3 inflammasome via cytotoxins and TLR/nucleotide-binding oligomerization domain-mediated NF-kappa B signaling.J Immunol. 2010 May 1;184(9):5287-97. doi: 10.4049/jimmunol.0903536. Epub 2010 Mar 26. J Immunol. 2010. PMID: 20348425
-
Regulation and Function of the Nucleotide Binding Domain Leucine-Rich Repeat-Containing Receptor, Pyrin Domain-Containing-3 Inflammasome in Lung Disease.Am J Respir Cell Mol Biol. 2016 Feb;54(2):151-60. doi: 10.1165/rcmb.2015-0231TR. Am J Respir Cell Mol Biol. 2016. PMID: 26418144 Free PMC article. Review.
Cited by
-
Radiation takes its Toll.Cancer Lett. 2015 Nov 28;368(2):238-45. doi: 10.1016/j.canlet.2015.03.031. Epub 2015 Mar 25. Cancer Lett. 2015. PMID: 25819030 Free PMC article. Review.
-
Differential expression of inflammasomes in lung cancer cell lines and tissues.Tumour Biol. 2015 Sep;36(10):7501-13. doi: 10.1007/s13277-015-3473-4. Epub 2015 Apr 25. Tumour Biol. 2015. PMID: 25910707
-
ROS-Mediated NLRP3 Inflammasome Activation in Brain, Heart, Kidney, and Testis Ischemia/Reperfusion Injury.Oxid Med Cell Longev. 2016;2016:2183026. doi: 10.1155/2016/2183026. Epub 2016 Apr 5. Oxid Med Cell Longev. 2016. PMID: 27127546 Free PMC article. Review.
-
Prdx6 Regulates Nlrp3 Inflammasome Activation-Driven Inflammatory Response in Lens Epithelial Cells.Int J Mol Sci. 2023 Nov 13;24(22):16276. doi: 10.3390/ijms242216276. Int J Mol Sci. 2023. PMID: 38003466 Free PMC article.
-
Covalent Targeting As a Common Mechanism for Inhibiting NLRP3 Inflammasome Assembly.ACS Chem Biol. 2024 Feb 16;19(2):254-265. doi: 10.1021/acschembio.3c00330. Epub 2024 Jan 10. ACS Chem Biol. 2024. PMID: 38198472 Free PMC article.
References
-
- Takeda K, Kaisho T, Akira S. Toll-like receptors. Annual review of immunology. 2003;21:335–376. - PubMed
-
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. - PubMed
-
- Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008;20:17–22. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annual review of immunology. 2009;27:229–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI083713/AI/NIAID NIH HHS/United States
- R01 AR055398/AR/NIAMS NIH HHS/United States
- R01 AI067497/AI/NIAID NIH HHS/United States
- AI-067497/AI/NIAID NIH HHS/United States
- R56 AI067497/AI/NIAID NIH HHS/United States
- AR055398/AR/NIAMS NIH HHS/United States
- R01 AI083713-02/AI/NIAID NIH HHS/United States
- R01 AI083641-01/AI/NIAID NIH HHS/United States
- R01 AG014357/AG/NIA NIH HHS/United States
- AG14357/AG/NIA NIH HHS/United States
- R37 AI067497/AI/NIAID NIH HHS/United States
- R01 AI067497-04/AI/NIAID NIH HHS/United States
- AI-065483/AI/NIAID NIH HHS/United States
- R01 AI065483/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

