Deconvolution of blood microarray data identifies cellular activation patterns in systemic lupus erythematosus
- PMID: 19568420
- PMCID: PMC2699551
- DOI: 10.1371/journal.pone.0006098
Deconvolution of blood microarray data identifies cellular activation patterns in systemic lupus erythematosus
Abstract
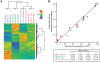
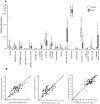
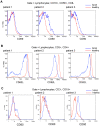
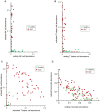
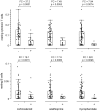
Systemic Lupus Erythematosus (SLE) is a systemic autoimmune disease with a complex spectrum of cellular and molecular characteristics including several dramatic changes in the populations of peripheral leukocytes. These changes include general leukopenia, activation of B and T cells, and maturation of granulocytes. The manifestation of SLE in peripheral blood is central to the disease but is incompletely understood. A technique for rigorously characterizing changes in mixed populations of cells, microarray expression deconvolution, has been applied to several areas of biology but not to SLE or to blood. Here we demonstrate that microarray expression deconvolution accurately quantifies the constituents of real blood samples and mixtures of immune-derived cell lines. We characterize a broad spectrum of peripheral leukocyte cell types and states in SLE to uncover novel patterns including: specific activation of NK and T helper lymphocytes, relationships of these patterns to each other, and correlations to clinical variables and measures. The expansion and activation of monocytes, NK cells, and T helper cells in SLE at least partly underlie this disease's prominent interferon signature. These and other patterns of leukocyte dynamics uncovered here correlate with disease severity and treatment, suggest potential new treatments, and extend our understanding of lupus pathology as a complex autoimmune disease involving many arms of the immune system.
Conflict of interest statement
Figures
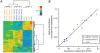


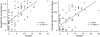
Similar articles
-
Two systemic lupus erythematosus (SLE) global disease activity indexes--the SLE Disease Activity Index and the Systemic Lupus Activity Measure--demonstrate different correlations with activation of peripheral blood CD4+ T cells.Hum Immunol. 2011 Dec;72(12):1160-7. doi: 10.1016/j.humimm.2011.08.005. Epub 2011 Aug 26. Hum Immunol. 2011. PMID: 21906646
-
Increased Concentrations of Circulating Soluble MHC Class I-Related Chain A (sMICA) and sMICB and Modulation of Plasma Membrane MICA Expression: Potential Mechanisms and Correlation With Natural Killer Cell Activity in Systemic Lupus Erythematosus.Front Immunol. 2021 May 3;12:633658. doi: 10.3389/fimmu.2021.633658. eCollection 2021. Front Immunol. 2021. PMID: 34012432 Free PMC article.
-
Low-density granulocytes activate T cells and demonstrate a non-suppressive role in systemic lupus erythematosus.Ann Rheum Dis. 2019 Jul;78(7):957-966. doi: 10.1136/annrheumdis-2018-214620. Epub 2019 Apr 30. Ann Rheum Dis. 2019. PMID: 31040119 Free PMC article.
-
Dysregulated Lymphoid Cell Populations in Mouse Models of Systemic Lupus Erythematosus.Clin Rev Allergy Immunol. 2017 Oct;53(2):181-197. doi: 10.1007/s12016-017-8605-8. Clin Rev Allergy Immunol. 2017. PMID: 28500565 Review.
-
[Role of T lymphocytes in systemic lupus erythematosus].Ann Med Interne (Paris). 1990;141(3):208-12. Ann Med Interne (Paris). 1990. PMID: 2195945 Review. French.
Cited by
-
An optimized pipeline for high-throughput bulk RNA-Seq deconvolution illustrates the impact of obesity and weight loss on cell composition of human adipose tissue.bioRxiv [Preprint]. 2024 Sep 25:2024.09.23.614489. doi: 10.1101/2024.09.23.614489. bioRxiv. 2024. PMID: 39386599 Free PMC article. Preprint.
-
Deconvolution from bulk gene expression by leveraging sample-wise and gene-wise similarities and single-cell RNA-Seq data.BMC Genomics. 2024 Sep 18;25(1):875. doi: 10.1186/s12864-024-10728-x. BMC Genomics. 2024. PMID: 39294558 Free PMC article.
-
Estimating cell-type-specific gene co-expression networks from bulk gene expression data with an application to Alzheimer's disease.J Am Stat Assoc. 2024;119(546):811-824. doi: 10.1080/01621459.2023.2297467. Epub 2024 Jan 31. J Am Stat Assoc. 2024. PMID: 39280354
-
Clonal associations between lymphocyte subsets and functional states in rheumatoid arthritis synovium.Nat Commun. 2024 Jun 11;15(1):4991. doi: 10.1038/s41467-024-49186-0. Nat Commun. 2024. PMID: 38862501 Free PMC article.
-
CATD: a reproducible pipeline for selecting cell-type deconvolution methods across tissues.Bioinform Adv. 2024 Mar 23;4(1):vbae048. doi: 10.1093/bioadv/vbae048. eCollection 2024. Bioinform Adv. 2024. PMID: 38638280 Free PMC article.
References
-
- Rivero SJ, Diaz-Jouanen E, Alarcon-Segovia D. Lymphopenia in systemic lupus erythematosus. Clinical, diagnostic, and prognostic significance. Arthritis Rheum. 1978;21:295–305. - PubMed
-
- Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. - PubMed
-
- Gaipl US, Voll RE, Sheriff A, Franz S, Kalden JR, et al. Impaired clearance of dying cells in systemic lupus erythematosus. Autoimmun Rev. 2005;4:189–194. - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

