Bovine glycomacropeptide induces cytokine production in human monocytes through the stimulation of the MAPK and the NF-kappaB signal transduction pathways
- PMID: 19558546
- PMCID: PMC2743842
- DOI: 10.1111/j.1476-5381.2009.00195.x
Bovine glycomacropeptide induces cytokine production in human monocytes through the stimulation of the MAPK and the NF-kappaB signal transduction pathways
Abstract
Background and purpose: Bovine glycomacropeptide (BGMP) is a natural milk peptide that is produced naturally in the gastrointestinal tract during digestion. Glycomacropepide has intestinal anti-inflammatory activity, but the mechanism of action is unknown. Here we have characterized the effects of BGMP on monocytes.
Experimental approach: We have used human THP-1 cells as an in vitro monocyte model. The effect of BGMP on the secretion of tumour necrosis factor (TNF), interleukin (IL)-1beta and IL-8 was assessed, as well as the involvement of the NF-kappaB and MAP kinase signalling pathways. The stimulatory effect of BGMP was also tested in human peripheral blood monocytes.
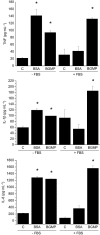
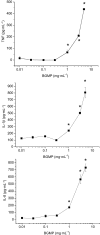
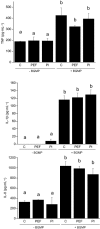
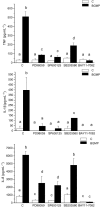
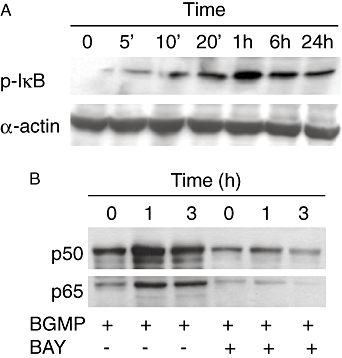
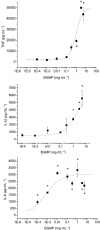
Key results: BGMP up-regulated the secretion of TNF, IL-1beta and IL-8 in a concentration-dependent fashion. The biological activity was exerted by the intact peptide, because cytokine secretion was not affected by protease inhibitors. The secretion of IL-8 and specially TNF and IL-1beta was blocked by PD98059, SP600125, SB203580 and Bay11-7082, suggesting the involvement of the MAP kinases p38, c-Jun N-terminal kinase and ERK and particularly the NF-kappaB pathway, although IL-8 secretion was independent of p38. BGMP was shown to elicit the phosphorylation of IkappaB-alpha and the nuclear translocation of the NF-kappaB subunits p50 and p65. The effect of BGMP on cytokine secretion was validated in human primary blood monocytes.
Conclusions and implications: BGMP stimulates human monocytes, operating via MAP kinase and NF-kappaB pathways. BGMP may exert an indirect intestinal anti-inflammatory effect by potentiating host defences against invading microorganisms.
Figures
Similar articles
-
(E)-3-(3,4-Dimethoxyphenyl)-1-(5-hydroxy-2,2-dimethyl-2H-chromen-6-yl)prop-2-en-1-one ameliorates the collagen-arthritis via blocking ERK/JNK and NF-κB signaling pathway.Int Immunopharmacol. 2013 Dec;17(4):1125-33. doi: 10.1016/j.intimp.2013.10.001. Epub 2013 Oct 14. Int Immunopharmacol. 2013. PMID: 24135236
-
Buthionine sulfoximine, an inhibitor of glutathione biosynthesis, induces expression of soluble epoxide hydrolase and markers of cellular hypertrophy in a rat cardiomyoblast cell line: roles of the NF-κB and MAPK signaling pathways.Free Radic Biol Med. 2015 May;82:1-12. doi: 10.1016/j.freeradbiomed.2015.01.005. Epub 2015 Jan 19. Free Radic Biol Med. 2015. PMID: 25614461
-
Anti-inflammatory effects of moxifloxacin on activated human monocytic cells: inhibition of NF-kappaB and mitogen-activated protein kinase activation and of synthesis of proinflammatory cytokines.Antimicrob Agents Chemother. 2004 Jun;48(6):1974-82. doi: 10.1128/AAC.48.6.1974-1982.2004. Antimicrob Agents Chemother. 2004. PMID: 15155187 Free PMC article.
-
[Interaction between p38 mitogen-activated protein kinase signal transduction pathway and NF-kappaB/IkappaB system on the proinflammatory cytokines release after burn trauma].Zhonghua Wai Ke Za Zhi. 2006 Apr 1;44(7):492-5. Zhonghua Wai Ke Za Zhi. 2006. PMID: 16772089 Chinese.
-
IL-32θ: a recently identified anti-inflammatory variant of IL-32 and its preventive role in various disorders and tumor suppressor activity.Am J Transl Res. 2017 Nov 15;9(11):4726-4737. eCollection 2017. Am J Transl Res. 2017. PMID: 29218075 Free PMC article. Review.
Cited by
-
Food derived bioactive peptides and intestinal barrier function.Int J Mol Sci. 2014 Dec 9;15(12):22857-73. doi: 10.3390/ijms151222857. Int J Mol Sci. 2014. PMID: 25501338 Free PMC article. Review.
-
Glycomacropeptide Bioactivity and Health: A Review Highlighting Action Mechanisms and Signaling Pathways.Nutrients. 2019 Mar 12;11(3):598. doi: 10.3390/nu11030598. Nutrients. 2019. PMID: 30870995 Free PMC article. Review.
-
Molecular mechanisms by which casein glycomacropeptide maintains internal homeostasis in mice with experimental ulcerative colitis.PLoS One. 2017 Jul 10;12(7):e0181075. doi: 10.1371/journal.pone.0181075. eCollection 2017. PLoS One. 2017. PMID: 28700735 Free PMC article.
-
De Novo Hepatocellular Carcinoma in Hepatitis C-Related Cirrhosis: Are Advanced Glycation End Products a Key Driver?Front Cell Infect Microbiol. 2021 Oct 1;11:662431. doi: 10.3389/fcimb.2021.662431. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34660332 Free PMC article.
-
Immunoregulatory Effects of Porcine Plasma Protein Concentrates on Rat Intestinal Epithelial Cells and Splenocytes.Animals (Basel). 2021 Mar 13;11(3):807. doi: 10.3390/ani11030807. Animals (Basel). 2021. PMID: 33805697 Free PMC article.
References
-
- Aimutis WR. Bioactive properties of milk proteins with particular focus on anticariogenesis. J Nutr. 2004;134:989S–995S. - PubMed
-
- Bal dit Sollier C, Drouet L, Pignaud G, Chevallier C, Caen J, Fiat AM, et al. Effect of kappa-casein split peptides on platelet aggregation and on thrombus formation in the guinea-pig. Thromb Res. 1996;81:427–437. - PubMed
-
- Bamias G, Cominelli F. Immunopathogenesis of inflammatory bowel disease: current concepts. Curr Opin Gastroenterol. 2007;23:365–359. - PubMed
-
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

