Toll-like receptor 4 deficiency increases disease and mortality after mouse hepatitis virus type 1 infection of susceptible C3H mice
- PMID: 19553337
- PMCID: PMC2738158
- DOI: 10.1128/JVI.01857-08
Toll-like receptor 4 deficiency increases disease and mortality after mouse hepatitis virus type 1 infection of susceptible C3H mice
Abstract
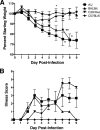
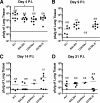
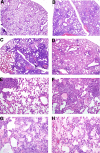
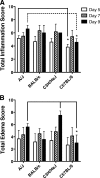
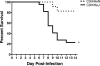
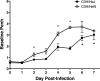
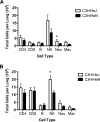
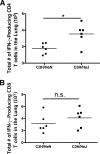
Severe acute respiratory syndrome (SARS) is characterized by substantial acute pulmonary inflammation with a high mortality rate. Despite the identification of SARS coronavirus (SARS-CoV) as the etiologic agent of SARS, a thorough understanding of the underlying disease pathogenesis has been hampered by the lack of a suitable animal model that recapitulates the human disease. Intranasal (i.n.) infection of A/J mice with the CoV mouse hepatitis virus strain 1 (MHV-1) induces an acute respiratory disease with a high lethality rate that shares several pathological similarities with SARS-CoV infection in humans. In this study, we examined virus replication and the character of pulmonary inflammation induced by MHV-1 infection in susceptible (A/J, C3H/HeJ, and BALB/c) and resistant (C57BL/6) strains of mice. Virus replication and distribution did not correlate with the relative susceptibilities of A/J, BALB/c, C3H/HeJ, and C57BL/6 mice. In order to further define the role of the host genetic background in influencing susceptibility to MHV-1-induced disease, we examined 14 different inbred mouse strains. BALB.B and BALB/c mice exhibited MHV-1-induced weight loss, whereas all other strains of H-2(b) and H-2(d) mice did not show any signs of disease following MHV-1 infection. H-2(k) mice demonstrated moderate susceptibility, with C3H/HeJ mice exhibiting the most severe disease. C3H/HeJ mice harbor a natural mutation in the gene that encodes Toll-like receptor 4 (TLR4) that disrupts TLR4 signaling. C3H/HeJ mice exhibit enhanced morbidity and mortality following i.n. MHV-1 infection compared to wild-type C3H/HeN mice. Our results indicate that TLR4 plays an important role in respiratory CoV pathogenesis.
Figures

Similar articles
-
Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in A/J mice.J Virol. 2006 Nov;80(21):10382-94. doi: 10.1128/JVI.00747-06. J Virol. 2006. PMID: 17041219 Free PMC article.
-
Protective and pathologic roles of the immune response to mouse hepatitis virus type 1: implications for severe acute respiratory syndrome.J Virol. 2009 Sep;83(18):9258-72. doi: 10.1128/JVI.00355-09. Epub 2009 Jul 1. J Virol. 2009. PMID: 19570864 Free PMC article.
-
Lack of association between the Tlr4 (Lpsd/Lpsd) genotype and increased susceptibility to Escherichia coli bladder infections in female C3H/HeJ mice.mBio. 2011 May 31;2(3):e00094-11. doi: 10.1128/mBio.00094-11. Print 2011. mBio. 2011. PMID: 21628500 Free PMC article.
-
Insights into coronavirus immunity taught by the murine coronavirus.Eur J Immunol. 2021 May;51(5):1062-1070. doi: 10.1002/eji.202048984. Epub 2021 Mar 22. Eur J Immunol. 2021. PMID: 33687066 Free PMC article. Review.
-
Murine-β-coronavirus-induced neuropathogenesis sheds light on CNS pathobiology of SARS-CoV2.J Neurovirol. 2021 Apr;27(2):197-216. doi: 10.1007/s13365-021-00945-5. Epub 2021 Feb 5. J Neurovirol. 2021. PMID: 33547593 Free PMC article. Review.
Cited by
-
A GABA-receptor agonist reduces pneumonitis severity, viral load, and death rate in SARS-CoV-2-infected mice.Front Immunol. 2022 Oct 25;13:1007955. doi: 10.3389/fimmu.2022.1007955. eCollection 2022. Front Immunol. 2022. PMID: 36389819 Free PMC article.
-
GABA administration prevents severe illness and death following coronavirus infection in mice.bioRxiv [Preprint]. 2020 Oct 4:2020.10.04.325423. doi: 10.1101/2020.10.04.325423. bioRxiv. 2020. Update in: Viruses. 2021 May 23;13(6):966. doi: 10.3390/v13060966 PMID: 33024975 Free PMC article. Updated. Preprint.
-
Impact of COVID-19 outbreak in an Italian cohort of patients with systemic sclerosis.Ther Adv Musculoskelet Dis. 2020 Sep 24;12:1759720X20953356. doi: 10.1177/1759720X20953356. eCollection 2020. Ther Adv Musculoskelet Dis. 2020. PMID: 33029203 Free PMC article.
-
Innate immune sensing of coronavirus and viral evasion strategies.Exp Mol Med. 2021 May;53(5):723-736. doi: 10.1038/s12276-021-00602-1. Epub 2021 May 6. Exp Mol Med. 2021. PMID: 33953325 Free PMC article. Review.
-
Priming With Rhinovirus Protects Mice Against a Lethal Pulmonary Coronavirus Infection.Front Immunol. 2022 May 30;13:886611. doi: 10.3389/fimmu.2022.886611. eCollection 2022. Front Immunol. 2022. PMID: 35711419 Free PMC article.
References
-
- De Albuquerque, N., E. Baig, X. Ma, J. Zhang, W. He, A. Rowe, M. Habal, M. Liu, I. Shalev, G. P. Downey, R. Gorczynski, J. Butany, J. Leibowitz, S. R. Weiss, I. D. McGilvray, M. J. Phillips, E. N. Fish, and G. A. Levy. 2006. Murine hepatitis virus strain 1 produces a clinically relevant model of severe acute respiratory syndrome in a/j mice. J. Virol. 8010382-10394. - PMC - PubMed
-
- Duniho, S. M., J. Martin, J. S. Forster, M. B. Cascio, T. S. Moran, L. B. Carpin, and A. M. Sciuto. 2002. Acute changes in lung histopathology and bronchoalveolar lavage parameters in mice exposed to the choking agent gas phosgene. Toxicol. Pathol. 30339-349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

