Nucleotide analogue prodrug tenofovir disoproxil enhances lymphoid cell loading following oral administration in monkeys
- PMID: 19545170
- PMCID: PMC2777719
- DOI: 10.1021/mp900036s
Nucleotide analogue prodrug tenofovir disoproxil enhances lymphoid cell loading following oral administration in monkeys
Abstract
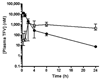
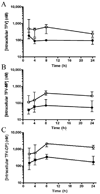
The antiviral drug tenofovir (TFV) is orally administered as the fumarate salt of its disoproxil prodrug (TFV disoproxil fumarate (TDF)). TFV is a dianion at physiological pH and, as a result, has poor lipid membrane permeability. Administration of the lipophilic and cell-permeable prodrug, TFV disoproxil, enhances the oral absorption of TFV. In order to determine whether oral administration of TDF also increases distribution to sites of viral infection, the plasma and circulating lymphoid cell pharmacokinetics of TFV and its phosphorylated metabolites were assessed following a single oral TDF or subcutaneous TFV administration at doses yielding equivalent plasma exposures to TFV in macaques. Despite TFV disoproxil's lack of plasma stability and undetectable levels in the first plasma samples taken, oral administration of TDF resulted in 7.9-fold higher peripheral blood mononuclear cell exposures to the active metabolite, TFV-diphosphate. The apparent plasma terminal half-life (t(1/2)) of TFV was also longer following oral TDF relative to subcutaneous TFV administration (median t(1/2) of 15.3 and 3.9 h, respectively), suggesting broader distribution to cells and tissues outside of the central plasma compartment. In conclusion, the disoproxil pro-moiety enhances not only the oral absorption of TFV but also tissue and lymphoid cell loading. These results illustrate that administration of even a fleeting prodrug can increase target tissue loading and give valuable insight for future prodrug development.
Figures

Similar articles
-
Natural substrate concentrations can modulate the prophylactic efficacy of nucleotide HIV reverse transcriptase inhibitors.J Virol. 2011 Jul;85(13):6610-7. doi: 10.1128/JVI.00311-11. Epub 2011 Apr 27. J Virol. 2011. PMID: 21525346 Free PMC article.
-
Single dose pharmacokinetics of oral tenofovir in plasma, peripheral blood mononuclear cells, colonic tissue, and vaginal tissue.AIDS Res Hum Retroviruses. 2013 Nov;29(11):1443-50. doi: 10.1089/aid.2013.0044. Epub 2013 May 29. AIDS Res Hum Retroviruses. 2013. PMID: 23600365 Free PMC article. Clinical Trial.
-
A multi-compartment single and multiple dose pharmacokinetic comparison of rectally applied tenofovir 1% gel and oral tenofovir disoproxil fumarate.PLoS One. 2014 Oct 28;9(10):e106196. doi: 10.1371/journal.pone.0106196. eCollection 2014. PLoS One. 2014. PMID: 25350119 Free PMC article. Clinical Trial.
-
Tenofovir alafenamide: A novel prodrug of tenofovir for the treatment of Human Immunodeficiency Virus.Antiviral Res. 2016 Jan;125:63-70. doi: 10.1016/j.antiviral.2015.11.009. Epub 2015 Nov 27. Antiviral Res. 2016. PMID: 26640223 Review.
-
Emtricitabine/tenofovir in the treatment of HIV infection: current PK/PD evaluation.Expert Opin Drug Metab Toxicol. 2012 Oct;8(10):1305-14. doi: 10.1517/17425255.2012.714367. Epub 2012 Sep 4. Expert Opin Drug Metab Toxicol. 2012. PMID: 22943210 Review.
Cited by
-
Improved pharmacokinetics of tenofovir ester prodrugs strengthened the inhibition of HBV replication and the rebalance of hepatocellular metabolism in preclinical models.Front Pharmacol. 2022 Aug 29;13:932934. doi: 10.3389/fphar.2022.932934. eCollection 2022. Front Pharmacol. 2022. PMID: 36105197 Free PMC article.
-
Simultaneous quantification of intracellular natural and antiretroviral nucleosides and nucleotides by liquid chromatography-tandem mass spectrometry.Anal Chem. 2010 Mar 1;82(5):1982-9. doi: 10.1021/ac902737j. Anal Chem. 2010. PMID: 20143781 Free PMC article.
-
Long-Acting Profile of 4 Drugs in 1 Anti-HIV Nanosuspension in Nonhuman Primates for 5 Weeks After a Single Subcutaneous Injection.J Pharm Sci. 2018 Jul;107(7):1787-1790. doi: 10.1016/j.xphs.2018.03.005. Epub 2018 Mar 13. J Pharm Sci. 2018. PMID: 29548975 Free PMC article.
-
Recent developments of nanotherapeutics for targeted and long-acting, combination HIV chemotherapy.Eur J Pharm Biopharm. 2019 May;138:75-91. doi: 10.1016/j.ejpb.2018.04.014. Epub 2018 Apr 17. Eur J Pharm Biopharm. 2019. PMID: 29678735 Free PMC article. Review.
-
Population Pharmacokinetics of Tenofovir in Pregnant and Postpartum Women Using Tenofovir Disoproxil Fumarate.Antimicrob Agents Chemother. 2021 Feb 17;65(3):e02168-20. doi: 10.1128/AAC.02168-20. Print 2021 Feb 17. Antimicrob Agents Chemother. 2021. PMID: 33318014 Free PMC article.
References
-
- Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 1998;42:612–617. - PMC - PubMed
-
- Barditch-Crovo P, Deeks SG, Collier A, Safrin S, Coakley DF, Miller M, Kearney BP, Coleman RL, Lamy PD, Kahn JO, McGowan I, Lietman PS. Phase i/ii trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 2001;45:2733–2739. - PMC - PubMed
-
- Kearney BP, Flaherty JF, Shah J. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 2004;43:595–612. - PubMed
-
- Lee WA, He GX, Eisenberg E, Cihlar T, Swaminathan S, Mulato A, Cundy KC. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 2005;49:1898–1906. - PMC - PubMed
-
- van Gelder J, Deferme S, Naesens L, De Clercq E, van den Mooter G, Kinget R, Augustijns P. Intestinal absorption enhancement of the ester prodrug tenofovir disoproxil fumarate through modulation of the biochemical barrier by defined ester mixtures. Drug Metab. Dispos. 2002;30:924–930. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

