Randomized controlled trial of feeding a concentrated formula to infants born to women infected by human immunodeficiency virus
- PMID: 19543114
- PMCID: PMC3934421
- DOI: 10.1097/MPG.0b013e3181928937
Randomized controlled trial of feeding a concentrated formula to infants born to women infected by human immunodeficiency virus
Abstract
Objective: We tested the hypothesis that concentrated formula (CF) begun within the first 2 weeks of life increases growth in infants born to human immunodeficiency virus (HIV)-infected mothers.
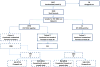
Materials and methods: HIV-exposed infants from the United States, the Bahamas, and Brazil were randomized in a double-blind, controlled trial to receive either a CF (87 kcal/100 mL [26 kcal/oz]) or a standard formula (SF; 67 kcal/100 mL [20 kcal/oz]) for 8 weeks. This article presents results for infants who were not determined to be HIV infected based on testing at 4 weeks. Primary outcomes were safety, tolerability, and growth in weight and length.
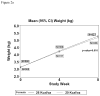
Results: Two thousand ninety-seven infants were enrolled, of whom 1998 were uninfected and had study formula dispensed. At weeks 4 and 8, uninfected infants receiving CF showed higher energy intake than those who were receiving SF (P < 0.001). By week 8, uninfected infants assigned to CF weighed more than infants receiving SF. There were no consistent differences in measures of tolerability, and rates of discontinuation or perceived formula intolerance were similar between treatment groups.
Conclusions: A CF is well tolerated and results in increased weight gain compared with SF. Until the HIV status of an infant is reliably determined, early introduction of a CF in HIV-exposed children may have beneficial effects on growth. The role of early nutritional intervention remains to be determined for individuals living in countries with endemic malnutrition for whom formula feeding is a viable option.
Figures

Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Growth and metabolism of infants born to women infected with human immunodeficiency virus and fed acidified whey-adapted starter formulas.Nutrition. 2008 Mar;24(3):203-11. doi: 10.1016/j.nut.2007.11.002. Epub 2007 Dec 21. Nutrition. 2008. PMID: 18160258 Clinical Trial.
-
Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial.Am J Clin Nutr. 2014 Apr;99(4):860-8. doi: 10.3945/ajcn.113.064295. Epub 2014 Feb 5. Am J Clin Nutr. 2014. PMID: 24500150 Clinical Trial.
-
Dilute versus full strength formula in exclusively formula-fed preterm or low birth weight infants.Cochrane Database Syst Rev. 2013 Nov 5;(11):CD007263. doi: 10.1002/14651858.CD007263.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2019 Jun 27;6:CD007263. doi: 10.1002/14651858.CD007263.pub3 PMID: 24194466 Updated. Review.
-
Formulas containing hydrolysed protein for prevention of allergy and food intolerance in infants.Cochrane Database Syst Rev. 2006 Oct 18;(4):CD003664. doi: 10.1002/14651858.CD003664.pub3. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2017 Mar 15;3:CD003664. doi: 10.1002/14651858.CD003664.pub4 PMID: 17054180 Updated. Review.
Cited by
-
Effects of postnatal interventions for the reduction of vertical HIV transmission on infant growth and non-HIV infections: a systematic review.J Int AIDS Soc. 2013 Dec 20;16(1):18865. doi: 10.7448/IAS.16.1.18865. J Int AIDS Soc. 2013. PMID: 24369738 Free PMC article. Review.
-
Effect of probiotic bacteria on microbial host defense, growth, and immune function in human immunodeficiency virus type-1 infection.Nutrients. 2011 Dec;3(12):1042-70. doi: 10.3390/nu3121042. Epub 2011 Dec 19. Nutrients. 2011. PMID: 22292110 Free PMC article.
References
-
- [Accessed 8 June 2008];UNAIDS AIDS epidemic update. 2006 Dec; Available at: http://www.unaids.org/en/HIV_data/epi2006.
-
- Johnson RP. How HIV Guts the Immune System. New Engl J Med. 2008;358(21):2287–9. - PubMed
-
- Iroha EO, Ezeaka VC, Akinsulie AO, Temiye EO, Adetifa IM. Maternal HIV infection and intrauterine growth: a prospective study in Lagos, Nigeria. West Afr J Med. 2007;26:121–5. - PubMed
-
- National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. OPRR Reports. Office of Protection from Research Risks; Washington, D.C: Apr 18, 1986. The Belmont report: ethical principles and guidelines for the protection of human subjects of research. 1979. - PubMed
-
- Code of Federal Regulations. Protection of human subjects. Office of Protection from Research Risks; Washington, D.C: 1992. Title 45 CFR Part 46, Protection of Human Subjects, revised June 18, 1991. OPRR Reports.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

