Paradoxical condensation of copper with elevated beta-amyloid in lipid rafts under cellular copper deficiency conditions: implications for Alzheimer disease
- PMID: 19542222
- PMCID: PMC2755914
- DOI: 10.1074/jbc.M109.019521
Paradoxical condensation of copper with elevated beta-amyloid in lipid rafts under cellular copper deficiency conditions: implications for Alzheimer disease
Abstract
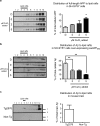
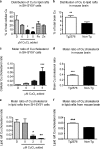
Redox-active copper is implicated in the pathogenesis of Alzheimer disease (AD), beta-amyloid peptide (Abeta) aggregation, and amyloid formation. Abeta.copper complexes have been identified in AD and catalytically oxidize cholesterol and lipid to generate H2O2 and lipid peroxides. The site and mechanism of this abnormality is not known. Growing evidence suggests that amyloidogenic processing of the beta-amyloid precursor protein (APP) occurs in lipid rafts, membrane microdomains enriched in cholesterol. beta- and gamma-secretases, and Abeta have been identified in lipid rafts in cultured cells, human and rodent brains, but the role of copper in lipid raft amyloidogenic processing is presently unknown. In this study, we found that copper modulates flotillin-2 association with cholesterol-rich lipid raft domains, and consequently Abeta synthesis is attenuated via copper-mediated inhibition of APP endocytosis. We also found that total cellular copper is associated inversely with lipid raft copper levels, so that under intracellular copper deficiency conditions, Abeta.copper complexes are more likely to form. This explains the paradoxical hypermetallation of Abeta with copper under tissue copper deficiency conditions in AD.
Figures
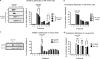

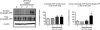
Similar articles
-
Membrane rafts in Alzheimer's disease beta-amyloid production.Biochim Biophys Acta. 2010 Aug;1801(8):860-7. doi: 10.1016/j.bbalip.2010.03.007. Epub 2010 Mar 18. Biochim Biophys Acta. 2010. PMID: 20303415 Free PMC article. Review.
-
Alzheimer disease Abeta production in the absence of S-palmitoylation-dependent targeting of BACE1 to lipid rafts.J Biol Chem. 2009 Feb 6;284(6):3793-803. doi: 10.1074/jbc.M808920200. Epub 2008 Dec 12. J Biol Chem. 2009. PMID: 19074428 Free PMC article.
-
Aβ promotes VDAC1 channel dephosphorylation in neuronal lipid rafts. Relevance to the mechanisms of neurotoxicity in Alzheimer's disease.Neuroscience. 2014 Oct 10;278:354-66. doi: 10.1016/j.neuroscience.2014.07.079. Epub 2014 Aug 26. Neuroscience. 2014. PMID: 25168729
-
Preferred Endocytosis of Amyloid Precursor Protein from Cholesterol-Enriched Lipid Raft Microdomains.Molecules. 2020 Nov 24;25(23):5490. doi: 10.3390/molecules25235490. Molecules. 2020. PMID: 33255194 Free PMC article.
-
Cholesterol and Lipid Rafts in the Biogenesis of Amyloid-β Protein and Alzheimer's Disease.Annu Rev Biophys. 2024 Jul;53(1):455-486. doi: 10.1146/annurev-biophys-062823-023436. Epub 2024 Jun 28. Annu Rev Biophys. 2024. PMID: 38382114 Review.
Cited by
-
An N-heterocyclic amine chelate capable of antioxidant capacity and amyloid disaggregation.ACS Chem Neurosci. 2012 Nov 21;3(11):919-27. doi: 10.1021/cn300060v. Epub 2012 Aug 31. ACS Chem Neurosci. 2012. PMID: 23173072 Free PMC article.
-
Copper in the brain and Alzheimer's disease.J Biol Inorg Chem. 2010 Jan;15(1):61-76. doi: 10.1007/s00775-009-0600-y. Epub 2009 Oct 28. J Biol Inorg Chem. 2010. PMID: 19862561 Review.
-
Low levels of copper disrupt brain amyloid-β homeostasis by altering its production and clearance.Proc Natl Acad Sci U S A. 2013 Sep 3;110(36):14771-6. doi: 10.1073/pnas.1302212110. Epub 2013 Aug 19. Proc Natl Acad Sci U S A. 2013. PMID: 23959870 Free PMC article.
-
Role of the P-Type ATPases, ATP7A and ATP7B in brain copper homeostasis.Front Aging Neurosci. 2013 Aug 23;5:44. doi: 10.3389/fnagi.2013.00044. eCollection 2013. Front Aging Neurosci. 2013. PMID: 23986700 Free PMC article.
-
Dissociation of ERK signalling inhibition from the anti-amyloidogenic action of synthetic ceramide analogues.Clin Sci (Lond). 2012 May;122(9):409-19. doi: 10.1042/CS20110257. Clin Sci (Lond). 2012. PMID: 22103431 Free PMC article.
References
-
- Lovell M. A., Robertson J. D., Teesdale W. J., Campbell J. L., Markesbery W. R. (1998) J. Neurol. Sci. 158, 47–52 - PubMed
-
- Miller L. M., Wang Q., Telivala T. P., Smith R. J., Lanzirotti A., Miklossy J. (2006) J. Struct. Biol. 155, 30–37 - PubMed
-
- Adlard P. A., Bush A. I. (2006) J. Alzheimers Dis. 10, 145–163 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

