The proapoptotic BH3-only, Bcl-2 family member, Puma is critical for acute ethanol-induced neuronal apoptosis
- PMID: 19535997
- PMCID: PMC2745204
- DOI: 10.1097/NEN.0b013e3181a9d524
The proapoptotic BH3-only, Bcl-2 family member, Puma is critical for acute ethanol-induced neuronal apoptosis
Abstract
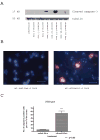
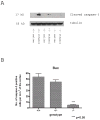
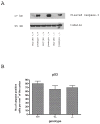
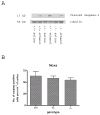
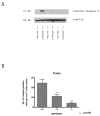
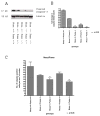
Synaptogenesis in humans occurs in the last trimester of gestation and in the first few years of life, whereas it occurs in the postnatal period in rodents. A single exposure of neonatal rodents to ethanol during this period evokes extensive neuronal apoptosis. Previous studies indicate that ethanol triggers the intrinsic apoptotic pathway in neurons, and that this requires the multi-BH domain, proapoptotic Bcl-2 family member Bax. To define the upstream regulators of this apoptotic pathway, we examined the possible roles of p53 and a subclass of proapoptotic Bcl-2 family members (i.e. the BH3 domain-only proteins) in neonatal wild-type and gene-targeted mice that lack these cell death inducers. Acute ethanol exposure produced greater caspase-3 activation and neuronal apoptosis in wild-type mice than in saline-treated littermate controls. Loss of p53-upregulated mediator of apoptosis (Puma) resulted in marked protection from ethanol-induced caspase-3 activation and apoptosis. Although Puma expression has been reported to be regulated by p53, p53-deficient mice exhibited a similar extent of ethanol-induced caspase-3 activation and neuronal apoptosis as wild-type mice. Mice deficient in other proapoptotic BH3-only proteins, including Noxa, Bim, or Hrk, showed no significant protection from ethanol-induced neuronal apoptosis. Collectively, these studies indicate a p53-independent, Bax- and Puma-dependent mechanism of neuronal apoptosis and identify Puma as a possible molecular target for inhibiting the effects of intrauterine ethanol exposure in humans.
Figures

Similar articles
-
BH3-only proapoptotic Bcl-2 family members Noxa and Puma mediate neural precursor cell death.J Neurosci. 2006 Jul 5;26(27):7257-64. doi: 10.1523/JNEUROSCI.0196-06.2006. J Neurosci. 2006. PMID: 16822983 Free PMC article.
-
Glucose induces pancreatic islet cell apoptosis that requires the BH3-only proteins Bim and Puma and multi-BH domain protein Bax.Diabetes. 2010 Mar;59(3):644-52. doi: 10.2337/db09-1151. Epub 2009 Dec 3. Diabetes. 2010. PMID: 19959756 Free PMC article.
-
Characterization of Puma-dependent and Puma-independent neuronal cell death pathways following prolonged proteasomal inhibition.Mol Cell Biol. 2010 Dec;30(23):5484-501. doi: 10.1128/MCB.00575-10. Epub 2010 Oct 4. Mol Cell Biol. 2010. PMID: 20921277 Free PMC article.
-
PUMA, a critical mediator of cell death--one decade on from its discovery.Cell Mol Biol Lett. 2012 Dec;17(4):646-69. doi: 10.2478/s11658-012-0032-5. Epub 2012 Sep 20. Cell Mol Biol Lett. 2012. PMID: 23001513 Free PMC article. Review.
-
PUMA, a potent killer with or without p53.Oncogene. 2008 Dec;27 Suppl 1(Suppl 1):S71-83. doi: 10.1038/onc.2009.45. Oncogene. 2008. PMID: 19641508 Free PMC article. Review.
Cited by
-
p53 Mediates Colistin-Induced Autophagy and Apoptosis in PC-12 Cells.Antimicrob Agents Chemother. 2016 Aug 22;60(9):5294-301. doi: 10.1128/AAC.00641-16. Print 2016 Sep. Antimicrob Agents Chemother. 2016. PMID: 27324771 Free PMC article.
-
Evaluation of the BH3-only protein Puma as a direct Bak activator.J Biol Chem. 2014 Jan 3;289(1):89-99. doi: 10.1074/jbc.M113.505701. Epub 2013 Nov 21. J Biol Chem. 2014. PMID: 24265320 Free PMC article.
-
Heterogeneity of p53 dependent genomic responses following ethanol exposure in a developmental mouse model of fetal alcohol spectrum disorder.PLoS One. 2017 Jul 19;12(7):e0180873. doi: 10.1371/journal.pone.0180873. eCollection 2017. PLoS One. 2017. PMID: 28723918 Free PMC article.
-
Neuronal cell life, death, and axonal degeneration as regulated by the BCL-2 family proteins.Cell Death Differ. 2021 Jan;28(1):108-122. doi: 10.1038/s41418-020-00654-2. Epub 2020 Nov 8. Cell Death Differ. 2021. PMID: 33162554 Free PMC article. Review.
-
Loss of tumor protein 53 protects against alcohol-induced facial malformations in mice and zebrafish.Alcohol Clin Exp Res. 2021 Oct;45(10):1965-1979. doi: 10.1111/acer.14688. Epub 2021 Sep 28. Alcohol Clin Exp Res. 2021. PMID: 34581462 Free PMC article.
References
-
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. - PubMed
-
- Jones KL, Smith DW, Ulleland CN, et al. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet. 1973;1:1267–71. - PubMed
-
- Clarren SK, Smith DW. The fetal alcohol syndrome. N Engl J Med. 1978;298:1063–67. - PubMed
-
- Swayze VW, Johnson VP, Hanson JW, et al. Magnetic resonance imaging of brain anomalies in fetal alcohol syndrome. Pediatrics. 1997;99:232–40. - PubMed
-
- Streissguth AP, O’Malley K. Neuropsychiatric implications and long-term consequences of fetal alcohol spectrum disorders. Semin Clin Neuropsychiatry. 2000;5:177–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

