Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease
- PMID: 19535994
- PMCID: PMC2725359
- DOI: 10.1097/NEN.0b013e3181aacbe9
Brains with medial temporal lobe neurofibrillary tangles but no neuritic amyloid plaques are a diagnostic dilemma but may have pathogenetic aspects distinct from Alzheimer disease
Abstract
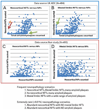
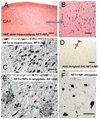
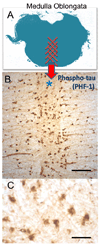
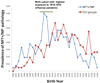
Brains that have many neurofibrillary tangles (NFTs) in medial temporal lobe structures (Braak stage III or IV) but no cortical neuritic plaques (NPs) may be a diagnostic dilemma; they also raise questions about the amyloid cascade hypothesis of Alzheimer disease (AD) in which NFT development is thought to occur downstream of the development of amyloid plaques. To determine the clinical, demographic, and biological factors related to NFT+/NP- cases, we analyzed 26 NFT+/NP- patient brains identified from the University of Kentucky AD Center autopsy cohort (n=502); most of these patients were at least 85 years old and lacked profound antemortem cognitive impairment. A subset of the cases had NFTs in the medulla oblongata. Aberrant trans-activator regulatory DNA-binding protein 43 immunohistochemical staining was seen in 5 of the 26 cases with the clinical diagnoses of AD or mild cognitive impairment. We also queried cases in the National Alzheimer's Coordinating Center Registry (n=5,108) and found 219 NFT+/NP- cases. Those patients had a relatively high likelihood of belonging to a birth cohort with the highest incidence of influenza infection during the 1918 to 1919 pandemic. This observation may link the pathogenesis in NFT+/NP- cases to encephalitis during childhood. We conclude that NFT+/NP- cases comprise approximately 5% of aged individuals in multiple data sets; these cases are not necessarily within the spectrum of AD.
Figures
Similar articles
-
Relative roles of plaques and tangles in the dementia of Alzheimer's disease: correlations using three sets of neuropathological criteria.Dementia. 1995 Jan-Feb;6(1):21-31. doi: 10.1159/000106918. Dementia. 1995. PMID: 7728216
-
Thal Amyloid Stages Do Not Significantly Impact the Correlation Between Neuropathological Change and Cognition in the Alzheimer Disease Continuum.J Neuropathol Exp Neurol. 2016 Jun;75(6):516-26. doi: 10.1093/jnen/nlw026. Epub 2016 Apr 22. J Neuropathol Exp Neurol. 2016. PMID: 27105663 Free PMC article.
-
Alzheimer's-type neuropathology in the precuneus is not increased relative to other areas of neocortex across a range of cognitive impairment.Neurosci Lett. 2009 Feb 6;450(3):336-9. doi: 10.1016/j.neulet.2008.11.006. Epub 2008 Nov 8. Neurosci Lett. 2009. PMID: 19010392 Free PMC article.
-
Neuropathology of Alzheimer's disease: a critical update.J Neural Transm Suppl. 1998;54:77-95. doi: 10.1007/978-3-7091-7508-8_8. J Neural Transm Suppl. 1998. PMID: 9850917 Review.
-
Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship.J Neuropathol Exp Neurol. 2009 Jan;68(1):1-14. doi: 10.1097/NEN.0b013e3181919a48. J Neuropathol Exp Neurol. 2009. PMID: 19104448 Free PMC article. Review.
Cited by
-
Limbic-predominant age-related TDP-43 encephalopathy differs from frontotemporal lobar degeneration.Brain. 2020 Sep 1;143(9):2844-2857. doi: 10.1093/brain/awaa219. Brain. 2020. PMID: 32830216 Free PMC article.
-
Novel human ABCC9/SUR2 brain-expressed transcripts and an eQTL relevant to hippocampal sclerosis of aging.J Neurochem. 2015 Sep;134(6):1026-39. doi: 10.1111/jnc.13202. Epub 2015 Jul 15. J Neurochem. 2015. PMID: 26115089 Free PMC article.
-
Primary age-related tauopathy (PART): a common pathology associated with human aging.Acta Neuropathol. 2014 Dec;128(6):755-66. doi: 10.1007/s00401-014-1349-0. Epub 2014 Oct 28. Acta Neuropathol. 2014. PMID: 25348064 Free PMC article.
-
PART, a distinct tauopathy, different from classical sporadic Alzheimer disease.Acta Neuropathol. 2015 May;129(5):757-62. doi: 10.1007/s00401-015-1407-2. Epub 2015 Mar 17. Acta Neuropathol. 2015. PMID: 25778618 Free PMC article. No abstract available.
-
Alzheimer's disease is not "brain aging": neuropathological, genetic, and epidemiological human studies.Acta Neuropathol. 2011 May;121(5):571-87. doi: 10.1007/s00401-011-0826-y. Epub 2011 Apr 24. Acta Neuropathol. 2011. PMID: 21516511 Free PMC article. Review.
References
-
- Armstrong RA. Plaques and tangles and the pathogenesis of Alzheimer's disease. Folia Neuropathol. 2006;44:1–11. - PubMed
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. - PubMed
-
- Braak H, Braak E. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278. discussion 8–84. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

