L-type calcium channel blockers exert an antiinflammatory effect by suppressing expression of plasminogen receptors on macrophages
- PMID: 19520970
- PMCID: PMC2745969
- DOI: 10.1161/CIRCRESAHA.109.200311
L-type calcium channel blockers exert an antiinflammatory effect by suppressing expression of plasminogen receptors on macrophages
Abstract
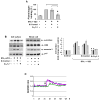
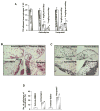
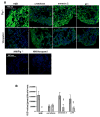
L-type Ca(2+) channel (LTCC) blockers, represented by amlodipine and verapamil, are widely used antihypertensive drugs that also have antiinflammatory activities. Plasminogen (Plg) is an important mediator of macrophage recruitment, and this role depends on its interaction with Plg receptors (Plg-Rs). Plg-Rs include histone 2B, alpha-enolase, annexin 2, and p11, all proteins which lack signal sequences for cell surface export. When human or murine monocytoid cells were induced to differentiate into macrophages, their Plg binding and Plg-R expression increased by 4-fold. These changes were suppressed by pretreatment with verapamil and amlodipine. Expression of the Ca(v)1.2 LTCC pore subunit was induced in differentiated macrophages, and siRNA against this subunit suppressed the upregulation of Plg binding and Plg-Rs. In vivo, amlodipine and verapamil suppressed peritoneal macrophage recruitment in response to thioglycollate by >60% at doses that did not affect blood pressure. In drug-treated animals, macrophages migrated into but not through the peritoneal membrane tissue and showed reduced surface expression of Plg-Rs. These findings demonstrate that Plg-R expression on macrophages is dependent on Ca(v)1.2 LTCC subunit expression. Suppression of Plg-Rs may contribute to the antiinflammatory effects of the widely used LTCC blockers.
Conflict of interest statement
Figures
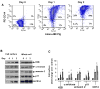
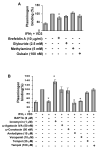
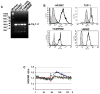
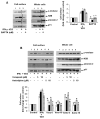
Comment in
-
L-type calcium channel antagonists and suppression of expression of plasminogen receptors: is the missing link the L-type calcium channel?Circ Res. 2009 Jul 17;105(2):112-3. doi: 10.1161/CIRCRESAHA.109.202028. Circ Res. 2009. PMID: 19608988 No abstract available.
Similar articles
-
A new function for old drugs.Cell Cycle. 2010 Feb 15;9(4):638-9. doi: 10.4161/cc.9.4.11016. Epub 2010 Feb 18. Cell Cycle. 2010. PMID: 20107310 Free PMC article. No abstract available.
-
L-type calcium channel antagonists and suppression of expression of plasminogen receptors: is the missing link the L-type calcium channel?Circ Res. 2009 Jul 17;105(2):112-3. doi: 10.1161/CIRCRESAHA.109.202028. Circ Res. 2009. PMID: 19608988 No abstract available.
-
Histone H2B as a functionally important plasminogen receptor on macrophages.Blood. 2007 Nov 15;110(10):3763-72. doi: 10.1182/blood-2007-03-079392. Epub 2007 Aug 9. Blood. 2007. PMID: 17690254 Free PMC article.
-
New insights into the role of Plg-RKT in macrophage recruitment.Int Rev Cell Mol Biol. 2014;309:259-302. doi: 10.1016/B978-0-12-800255-1.00005-3. Int Rev Cell Mol Biol. 2014. PMID: 24529725 Free PMC article. Review.
-
Plasminogen and its receptors as regulators of cardiovascular inflammatory responses.Trends Cardiovasc Med. 2010 May;20(4):120-4. doi: 10.1016/j.tcm.2010.10.002. Trends Cardiovasc Med. 2010. PMID: 21335281 Free PMC article. Review.
Cited by
-
Voltage-Gated Calcium Channels in Nonexcitable Tissues.Annu Rev Physiol. 2021 Feb 10;83:183-203. doi: 10.1146/annurev-physiol-031620-091043. Epub 2020 Oct 26. Annu Rev Physiol. 2021. PMID: 33106102 Free PMC article. Review.
-
A new function for old drugs.Cell Cycle. 2010 Feb 15;9(4):638-9. doi: 10.4161/cc.9.4.11016. Epub 2010 Feb 18. Cell Cycle. 2010. PMID: 20107310 Free PMC article. No abstract available.
-
The link between immunity and hypertension in the kidney and heart.Front Cardiovasc Med. 2023 Mar 9;10:1129384. doi: 10.3389/fcvm.2023.1129384. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36970367 Free PMC article. Review.
-
Targeting microglia L-type voltage-dependent calcium channels for the treatment of central nervous system disorders.J Neurosci Res. 2021 Jan;99(1):141-162. doi: 10.1002/jnr.24585. Epub 2020 Jan 29. J Neurosci Res. 2021. PMID: 31997405 Free PMC article. Review.
-
Association Between Prior Calcium Channel Blocker Use and Mortality in Septic Patients: A Meta-Analysis of Cohort Studies.Front Pharmacol. 2021 May 25;12:628825. doi: 10.3389/fphar.2021.628825. eCollection 2021. Front Pharmacol. 2021. PMID: 34113248 Free PMC article. Review.
References
-
- Ploplis VA, French EL, Carmeliet P, Collen D, Plow EF. Plasminogen deficiency differentially affects recruitment of inflammatory cell populations in mice. Blood. 1998;91:2005–9. - PubMed
-
- Plow EF, Herren T, Redlitz A, Miles LA, Hoover-Plow JL. The cell biology of the plasminogen system. FASEB J. 1995;9:939–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

