Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin
- PMID: 19516264
- PMCID: PMC2841955
- DOI: 10.1038/jid.2009.151
Imiquimod enhances IFN-gamma production and effector function of T cells infiltrating human squamous cell carcinomas of the skin
Abstract
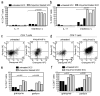
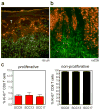
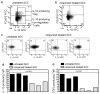
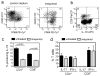
Squamous cell carcinomas (SCCs) are sun-induced skin cancers that are particularly numerous and aggressive in patients taking T-cell immunosuppressant medications. Imiquimod is a topical immune response modifier and Toll-like receptor 7 (TLR7) agonist that induces the immunological destruction of SCC and other skin cancers. TLR7 activation by imiquimod has pleiotropic effects on innate immune cells, but its effects on T cells remain largely uncharacterized. Because tumor destruction and formation of immunological memory are ultimately T-cell-mediated effects, we studied the effects of imiquimod therapy on effector T cells infiltrating human SCC. SCC treated with imiquimod before excision contained dense T-cell infiltrates associated with tumor cell apoptosis and histological evidence of tumor regression. Effector T cells from treated SCC produced more IFN-gamma, granzyme, and perforin and less IL-10 and transforming growth factor-beta (TGF-beta) than T cells from untreated tumors. Treatment of normal human skin with imiquimod induced activation of resident T cells and reduced IL-10 production but had no effect on IFN-gamma, perforin, or granzyme, suggesting that these latter effects arise from the recruitment of distinct populations of T cells into tumors. Thus, imiquimod stimulates tumor destruction by recruiting cutaneous effector T cells from blood and by inhibiting tonic anti-inflammatory signals within the tumor.
Conflict of interest statement
The authors state no conflict of interest.
Figures



Similar articles
-
Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells.J Exp Med. 2008 Sep 29;205(10):2221-34. doi: 10.1084/jem.20071190. Epub 2008 Sep 15. J Exp Med. 2008. PMID: 18794336 Free PMC article.
-
Imiquimod attenuates the growth of UVB-induced SCC in mice through Th1/Th17 cells.Mol Carcinog. 2013 Oct;52(10):760-9. doi: 10.1002/mc.21901. Epub 2012 Mar 16. Mol Carcinog. 2013. PMID: 22431065 Free PMC article.
-
Tumor-selective induction of apoptosis and the small-molecule immune response modifier imiquimod.J Natl Cancer Inst. 2003 Aug 6;95(15):1138-49. doi: 10.1093/jnci/djg016. J Natl Cancer Inst. 2003. PMID: 12902443
-
Topical imiquimod or fluorouracil therapy for basal and squamous cell carcinoma: a systematic review.Arch Dermatol. 2009 Dec;145(12):1431-8. doi: 10.1001/archdermatol.2009.291. Arch Dermatol. 2009. PMID: 20026854 Review.
-
Imiquimod - Its role in the treatment of cutaneous malignancies.Indian J Pharmacol. 2015 Jul-Aug;47(4):354-9. doi: 10.4103/0253-7613.161249. Indian J Pharmacol. 2015. PMID: 26288465 Free PMC article. Review.
Cited by
-
Effects of imiquimod and low-intensity laser (λ660 nm) in chemically induced oral carcinomas in hamster buccal pouch mucosa.Lasers Med Sci. 2013 May;28(3):1017-24. doi: 10.1007/s10103-012-1192-2. Epub 2012 Sep 1. Lasers Med Sci. 2013. PMID: 22941426
-
A TLR7 agonist strengthens T and NK cell function during BRAF-targeted therapy in a preclinical melanoma model.Int J Cancer. 2020 Mar 1;146(5):1409-1420. doi: 10.1002/ijc.32777. Epub 2019 Dec 4. Int J Cancer. 2020. PMID: 31702822 Free PMC article.
-
A combination of local inflammation and central memory T cells potentiates immunotherapy in the skin.J Immunol. 2012 Dec 15;189(12):5622-31. doi: 10.4049/jimmunol.1200709. Epub 2012 Nov 9. J Immunol. 2012. PMID: 23144496 Free PMC article.
-
Imiquimod induces apoptosis of squamous cell carcinoma (SCC) cells via regulation of A20.PLoS One. 2014 Apr 17;9(4):e95337. doi: 10.1371/journal.pone.0095337. eCollection 2014. PLoS One. 2014. PMID: 24743316 Free PMC article.
-
Topical imiquimod and cryotherapy in combination with systemic immunotherapy in unresectable stage IIIC melanoma.JAAD Case Rep. 2022 Jul 22;27:162-166. doi: 10.1016/j.jdcr.2022.07.019. eCollection 2022 Sep. JAAD Case Rep. 2022. PMID: 36097439 Free PMC article. No abstract available.
References
-
- Ambach A, Bonnekoh B, Nguyen M, Schon MP, Gollnick H. Imiquimod, a Toll-like receptor-7 agonist, induces perforin in cytotoxic T lymphocytes in vitro. Mol Immunol. 2004;40:1307–1314. - PubMed
-
- Berg D, Otley CC. Skin cancer in organ transplant recipients: Epidemiology, pathogenesis, and management. Journal of the American Academy of Dermatology. 2002;47:1–17. - PubMed
-
- Brown VL, Atkins CL, Ghali L, Cerio R, Harwood CA, Proby CM. Safety and efficacy of 5% imiquimod cream for the treatment of skin dysplasia in high-risk renal transplant recipients: randomized, double-blind, placebo-controlled trial. Archives of dermatology. 2005;141:985–993. - PubMed
-
- Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, Dowgiert RK, et al. A novel method for the isolation of skin resident T cells from normal and diseased human skin. The Journal of investigative dermatology. 2006;126:1059–1070. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

