Rapid immune recovery and graft-versus-host disease-like engraftment syndrome following adoptive transfer of Costimulated autologous T cells
- PMID: 19509133
- PMCID: PMC2828291
- DOI: 10.1158/1078-0432.CCR-09-0418
Rapid immune recovery and graft-versus-host disease-like engraftment syndrome following adoptive transfer of Costimulated autologous T cells
Abstract
Purpose: Previously, we showed that adoptive transfer of in vivo vaccine-primed and ex vivo (anti-CD3/anti-CD28) costimulated autologous T cells (ex-T) at day +12 after transplant increased CD4 and CD8 T-cell counts at day +42 and augmented vaccine-specific immune responses in patients with myeloma. Here, we investigated the safety and kinetics of T-cell recovery after infusing ex-T at day +2 after transplant.
Experimental design: In this phase I/II two-arm clinical trial, 50 patients with myeloma received autografts after high-dose melphalan followed by infusions of ex-T at day +2 after transplant. Patients also received pretransplant and posttransplant immunizations using a pneumococcal conjugate vaccine only (arm B; n = 24) or the pneumococcal conjugate vaccine plus an HLA-A2-restricted microltipeptide vaccine for HLA-A2(+) patients (arm A; n = 26).
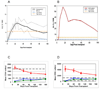
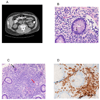
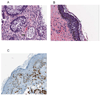
Results: The mean number of T cells infused was 4.26 x 10(10) (range, 1.59-5.0). At day 14 after transplant, the median CD3, CD4, and CD8 counts were 4,198, 1,545, and 2,858 cells/microL, respectively. Interleukin (IL)-6 and IL-15 levels increased early after transplant and IL-15 levels correlated significantly to day 14 T-cell counts. Robust vaccine-specific B- and T-cell responses were generated. T-cell infusions were well tolerated with no effect on hematopoietic recovery. Eight patients (16%) developed a T-cell "engraftment syndrome" characterized by diarrhea and fever that was clinically and histopathologically indistinguishable from grade 1 to 3 acute graft-versus-host disease (GVHD) of the gastrointestinal tract (seven patients) and/or grade 1 to 2 cutaneous GVHD (four patients).
Conclusions: Adoptive T-cell transfers achieve robust T-cell recovery early after transplant and induce moderate-to-severe autologous GVHD in a subset of patients.
Conflict of interest statement
Figures
Similar articles
-
Combination immunotherapy after ASCT for multiple myeloma using MAGE-A3/Poly-ICLC immunizations followed by adoptive transfer of vaccine-primed and costimulated autologous T cells.Clin Cancer Res. 2014 Mar 1;20(5):1355-65. doi: 10.1158/1078-0432.CCR-13-2817. Epub 2014 Feb 11. Clin Cancer Res. 2014. PMID: 24520093 Free PMC article. Clinical Trial.
-
Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma.Blood. 2011 Jan 20;117(3):788-97. doi: 10.1182/blood-2010-08-299396. Epub 2010 Oct 28. Blood. 2011. PMID: 21030558 Free PMC article. Clinical Trial.
-
Phase I trial of adoptive cell transfer with mixed-profile type-I/type-II allogeneic T cells for metastatic breast cancer.Clin Cancer Res. 2011 Nov 1;17(21):6878-87. doi: 10.1158/1078-0432.CCR-11-1579. Epub 2011 Sep 26. Clin Cancer Res. 2011. PMID: 21948234 Free PMC article. Clinical Trial.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Cellular immunotherapy for plasma cell myeloma.Bone Marrow Transplant. 2013 Nov;48(11):1377-86. doi: 10.1038/bmt.2013.54. Epub 2013 May 6. Bone Marrow Transplant. 2013. PMID: 23645169 Review.
Cited by
-
Allogeneic haematopoietic stem cell transplantation: individualized stem cell and immune therapy of cancer.Nat Rev Cancer. 2010 Mar;10(3):213-21. doi: 10.1038/nrc2804. Epub 2010 Feb 19. Nat Rev Cancer. 2010. PMID: 20168320 Review.
-
Long-term safety and activity of NY-ESO-1 SPEAR T cells after autologous stem cell transplant for myeloma.Blood Adv. 2019 Jul 9;3(13):2022-2034. doi: 10.1182/bloodadvances.2019000194. Blood Adv. 2019. PMID: 31289029 Free PMC article.
-
Engineering lymphocyte subsets: tools, trials and tribulations.Nat Rev Immunol. 2009 Oct;9(10):704-16. doi: 10.1038/nri2635. Nat Rev Immunol. 2009. PMID: 19859065 Free PMC article. Review.
-
Human CD19-Targeted Mouse T Cells Induce B Cell Aplasia and Toxicity in Human CD19 Transgenic Mice.Mol Ther. 2018 Jun 6;26(6):1423-1434. doi: 10.1016/j.ymthe.2018.04.006. Epub 2018 Apr 7. Mol Ther. 2018. PMID: 29735365 Free PMC article.
-
Efficient clinical scale gene modification via zinc finger nuclease-targeted disruption of the HIV co-receptor CCR5.Hum Gene Ther. 2013 Mar;24(3):245-58. doi: 10.1089/hum.2012.172. Epub 2013 Mar 6. Hum Gene Ther. 2013. PMID: 23360514 Free PMC article.
References
-
- Barlogie B, Jagannath S, Vesole DH, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–793. - PubMed
-
- Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. - PubMed
-
- Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. - PubMed
-
- Barlogie B, Tricot GJ, van Rhee F, et al. Long-term outcome results of the first tandem autotransplant trial for multiple myeloma. Br J Haematol. 2006;135:158–164. - PubMed
-
- Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87:1196–1198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

