Functionalized congeners of A3 adenosine receptor-selective nucleosides containing a bicyclo[3.1.0]hexane ring system
- PMID: 19499950
- PMCID: PMC3109436
- DOI: 10.1021/jm900426g
Functionalized congeners of A3 adenosine receptor-selective nucleosides containing a bicyclo[3.1.0]hexane ring system
Abstract
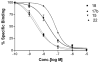
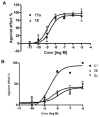
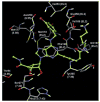
(N)-Methanocarba nucleosides containing bicyclo[3.1.0]hexane replacement of the ribose ring previously demonstrated selectivity as A(3) adenosine receptor (AR) agonists (5'-uronamides) or antagonists (5'-truncated). Here, these two series were modified in parallel at the adenine C2 position. N(6)-3-Chlorobenzyl-5'-N-methyluronamides derivatives with functionalized 2-alkynyl chains of varying length terminating in a reactive carboxylate, ester, or amine group were full, potent human A(3)AR agonists. Flexibility of chain substitution allowed the conjugation with a fluorescent cyanine dye (Cy5) and biotin, resulting in binding K(i) values of 17 and 36 nM, respectively. The distal end of the chain was predicted by homology modeling to bind at the A(3)AR extracellular regions. Corresponding l-nucleosides were nearly inactive in AR binding. In the 5'-truncated nucleoside series, 2-Cl analogues were more potent at A(3)AR than 2-H and 2-F, functional efficacy in adenylate cyclase inhibition varied, and introduction of a 2-alkynyl chain greatly reduced affinity. SAR parallels between the two series lost stringency at distal positions. The most potent and selective novel compounds were amine congener 15 (K(i) = 2.1 nM) and truncated partial agonist 22 (K(i) = 4.9 nM).
Figures



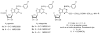
Similar articles
-
Selective A(3) adenosine receptor antagonists derived from nucleosides containing a bicyclo[3.1.0]hexane ring system.Bioorg Med Chem. 2008 Sep 15;16(18):8546-56. doi: 10.1016/j.bmc.2008.08.007. Epub 2008 Aug 7. Bioorg Med Chem. 2008. PMID: 18752961 Free PMC article.
-
(N)-methanocarba 2,N6-disubstituted adenine nucleosides as highly potent and selective A3 adenosine receptor agonists.J Med Chem. 2005 Mar 24;48(6):1745-58. doi: 10.1021/jm049580r. J Med Chem. 2005. PMID: 15771421 Free PMC article.
-
Structure-guided design of A(3) adenosine receptor-selective nucleosides: combination of 2-arylethynyl and bicyclo[3.1.0]hexane substitutions.J Med Chem. 2012 May 24;55(10):4847-60. doi: 10.1021/jm300396n. Epub 2012 May 16. J Med Chem. 2012. PMID: 22559880 Free PMC article.
-
Purine derivatives as ligands for A3 adenosine receptors.Curr Top Med Chem. 2005;5(13):1275-95. doi: 10.2174/156802605774463079. Curr Top Med Chem. 2005. PMID: 16305531 Free PMC article. Review.
-
Partial agonists for A(3) adenosine receptors.Curr Top Med Chem. 2004;4(8):855-62. doi: 10.2174/1568026043450989. Curr Top Med Chem. 2004. PMID: 15078216 Free PMC article. Review.
Cited by
-
2-Dialkynyl derivatives of (N)-methanocarba nucleosides: 'Clickable' A(3) adenosine receptor-selective agonists.Bioorg Med Chem. 2010 Jan 15;18(2):508-17. doi: 10.1016/j.bmc.2009.12.018. Epub 2009 Dec 11. Bioorg Med Chem. 2010. PMID: 20036562 Free PMC article.
-
Novel Alexa Fluor-488 labeled antagonist of the A(2A) adenosine receptor: Application to a fluorescence polarization-based receptor binding assay.Biochem Pharmacol. 2010 Aug 15;80(4):506-11. doi: 10.1016/j.bcp.2010.04.027. Epub 2010 May 11. Biochem Pharmacol. 2010. PMID: 20438717 Free PMC article.
-
Structural sweet spot for A1 adenosine receptor activation by truncated (N)-methanocarba nucleosides: receptor docking and potent anticonvulsant activity.J Med Chem. 2012 Sep 27;55(18):8075-90. doi: 10.1021/jm300965a. Epub 2012 Sep 10. J Med Chem. 2012. PMID: 22921089 Free PMC article.
-
Lighting up G protein-coupled purinergic receptors with engineered fluorescent ligands.Neuropharmacology. 2015 Nov;98:58-67. doi: 10.1016/j.neuropharm.2015.04.001. Epub 2015 Apr 16. Neuropharmacology. 2015. PMID: 25890205 Free PMC article. Review.
-
Synthesis and pharmacological characterization of [(125)I]MRS5127, a high affinity, selective agonist radioligand for the A3 adenosine receptor.Biochem Pharmacol. 2010 Apr 1;79(7):967-73. doi: 10.1016/j.bcp.2009.11.009. Epub 2009 Nov 14. Biochem Pharmacol. 2010. PMID: 19917269 Free PMC article.
References
-
- Jacobson KA, Klutz AM, Tosh DK, Ivanov AA, Preti D, Baraldi PG. Medicinal Chemistry of the A3 Adenosine Receptor: Agonists, Antagonists, and Receptor Engineering In HEP Adenosine Receptors in Health and Disease; Handbook of Experimental Pharmacology. Vol. 193. Springer; New York: 2009. - DOI - PMC - PubMed
-
- Gessi S, Merighi S, Varani K, Cattabriga E, Benini A, Mirandola P, Leung E, Mac Lennan S, Feo C, Baraldi S, Borea PA. Adenosine receptors in colon carcinoma tissues and colon tumoral cell lines: focus on the A3 adenosine subtype. J Cell Physiol. 2007;211:826–836. - PubMed
-
- Yamano K, Inoue M, Masaki S, Saki M, Ichimura M, Satoh M. Generation of adenosine A3 receptor functionally humanized mice for the evaluation of the human antagonists. Biochem Pharmacol. 2006;71:294–306. - PubMed
-
- Yang H, Avila MY, Peterson-Yantorno K, Coca-Prados M, Stone RA, Jacobson KA, Civan MM. The cross-species A3 adenosine-receptor antagonist MRS 1292 inhibits adenosine-triggered human nonpigmented ciliary epithelial cell fluid release and reduces mouse intraocular pressure. Curr Eye Res. 2005;30:747–754. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Research Materials
Miscellaneous

