Bile acid transporters
- PMID: 19498215
- PMCID: PMC2781307
- DOI: 10.1194/jlr.R900012-JLR200
Bile acid transporters
Abstract
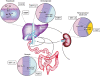
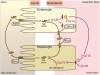
In liver and intestine, transporters play a critical role in maintaining the enterohepatic circulation and bile acid homeostasis. Over the past two decades, there has been significant progress toward identifying the individual membrane transporters and unraveling their complex regulation. In the liver, bile acids are efficiently transported across the sinusoidal membrane by the Na(+) taurocholate cotransporting polypeptide with assistance by members of the organic anion transporting polypeptide family. The bile acids are then secreted in an ATP-dependent fashion across the canalicular membrane by the bile salt export pump. Following their movement with bile into the lumen of the small intestine, bile acids are almost quantitatively reclaimed in the ileum by the apical sodium-dependent bile acid transporter. The bile acids are shuttled across the enterocyte to the basolateral membrane and effluxed into the portal circulation by the recently indentified heteromeric organic solute transporter, OSTalpha-OSTbeta. In addition to the hepatocyte and enterocyte, subgroups of these bile acid transporters are expressed by the biliary, renal, and colonic epithelium where they contribute to maintaining bile acid homeostasis and play important cytoprotective roles. This article will review our current understanding of the physiological role and regulation of these important carriers.
Figures
Similar articles
-
The heteromeric organic solute transporter alpha-beta, Ostalpha-Ostbeta, is an ileal basolateral bile acid transporter.J Biol Chem. 2005 Feb 25;280(8):6960-8. doi: 10.1074/jbc.M412752200. Epub 2004 Nov 24. J Biol Chem. 2005. PMID: 15563450 Free PMC article.
-
Role of the intestinal bile acid transporters in bile acid and drug disposition.Handb Exp Pharmacol. 2011;(201):169-203. doi: 10.1007/978-3-642-14541-4_4. Handb Exp Pharmacol. 2011. PMID: 21103970 Free PMC article. Review.
-
Targeting the Four Pillars of Enterohepatic Bile Salt Cycling; Lessons From Genetics and Pharmacology.Hepatology. 2021 Jun;73(6):2577-2585. doi: 10.1002/hep.31651. Epub 2021 May 24. Hepatology. 2021. PMID: 33222321 Free PMC article. Review.
-
Bile acid transporters: structure, function, regulation and pathophysiological implications.Pharm Res. 2007 Oct;24(10):1803-23. doi: 10.1007/s11095-007-9289-1. Epub 2007 Apr 3. Pharm Res. 2007. PMID: 17404808 Review.
-
Bile acid transporters in health and disease.Xenobiotica. 2008 Jul;38(7-8):1043-71. doi: 10.1080/00498250802040584. Xenobiotica. 2008. PMID: 18668439 Free PMC article. Review.
Cited by
-
The solute carrier family 10 (SLC10): beyond bile acid transport.Mol Aspects Med. 2013 Apr-Jun;34(2-3):252-69. doi: 10.1016/j.mam.2012.07.004. Mol Aspects Med. 2013. PMID: 23506869 Free PMC article. Review.
-
Evolution of substrate specificity for the bile salt transporter ASBT (SLC10A2).J Lipid Res. 2012 Aug;53(8):1535-42. doi: 10.1194/jlr.M025726. Epub 2012 Jun 5. J Lipid Res. 2012. PMID: 22669917 Free PMC article.
-
The alpha-glucosidase inhibitor miglitol increases hepatic CYP7A1 activity in association with altered short-chain fatty acid production in the gut of obese diabetic mice.Metabol Open. 2020 Jan 11;5:100024. doi: 10.1016/j.metop.2020.100024. eCollection 2020 Mar. Metabol Open. 2020. PMID: 32812937 Free PMC article.
-
Transcription of the Human Microsomal Epoxide Hydrolase Gene (EPHX1) Is Regulated by PARP-1 and Histone H1.2. Association with Sodium-Dependent Bile Acid Transport.PLoS One. 2015 May 20;10(5):e0125318. doi: 10.1371/journal.pone.0125318. eCollection 2015. PLoS One. 2015. PMID: 25992604 Free PMC article.
-
Immense Insulin Intestinal Uptake and Lymphatic Transport Using Bile Acid Conjugated Partially Uncapped Liposome.Mol Pharm. 2018 Oct 1;15(10):4756-4763. doi: 10.1021/acs.molpharmaceut.8b00708. Epub 2018 Aug 29. Mol Pharm. 2018. PMID: 30125508 Free PMC article.
References
-
- Alrefai W. A., Gill R. K. 2007. Bile acid transporters: structure, function, regulation and pathophysiological implications. Pharm. Res. 24: 1803–1823 - PubMed
-
- Trauner M., Boyer J. L. 2003. Bile salt transporters: molecular characterization, function, and regulation. Physiol. Rev. 83: 633–671 - PubMed
-
- Thomas C., Pellicciari R., Pruzanski M., Auwerx J., Schoonjans K. 2008. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 7: 678–693 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

