Molecular interactions in rotavirus assembly and uncoating seen by high-resolution cryo-EM
- PMID: 19487668
- PMCID: PMC2689313
- DOI: 10.1073/pnas.0904024106
Molecular interactions in rotavirus assembly and uncoating seen by high-resolution cryo-EM
Abstract
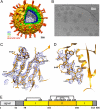
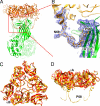
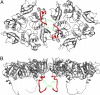
Rotaviruses, major causes of childhood gastroenteritis, are nonenveloped, icosahedral particles with double-strand RNA genomes. By the use of electron cryomicroscopy and single-particle reconstruction, we have visualized a rotavirus particle comprising the inner capsid coated with the trimeric outer-layer protein, VP7, at a resolution (4 A) comparable with that of X-ray crystallography. We have traced the VP7 polypeptide chain, including parts not seen in its X-ray crystal structure. The 3 well-ordered, 30-residue, N-terminal "arms" of each VP7 trimer grip the underlying trimer of VP6, an inner-capsid protein. Structural differences between free and particle-bound VP7 and between free and VP7-coated inner capsids may regulate mRNA transcription and release. The Ca(2+)-stabilized VP7 intratrimer contact region, which presents important neutralizing epitopes, is unaltered upon capsid binding.
Conflict of interest statement
Conflict of interest statement: E.C.S. and P.R.D. are employees and shareholders of Novartis Vaccines and Diagnostics, Inc.
Figures

Comment in
-
Single-particle cryoEM reconstructions: meeting the challenge.Proc Natl Acad Sci U S A. 2009 Jun 30;106(26):10398-9. doi: 10.1073/pnas.0905001106. Epub 2009 Jun 24. Proc Natl Acad Sci U S A. 2009. PMID: 19553214 Free PMC article. No abstract available.
Similar articles
-
Single-particle cryoEM reconstructions: meeting the challenge.Proc Natl Acad Sci U S A. 2009 Jun 30;106(26):10398-9. doi: 10.1073/pnas.0905001106. Epub 2009 Jun 24. Proc Natl Acad Sci U S A. 2009. PMID: 19553214 Free PMC article. No abstract available.
-
Projection structure of VP6, the rotavirus inner capsid protein, and comparison with bluetongue VP7.J Mol Biol. 1997 Sep 26;272(3):362-8. doi: 10.1006/jmbi.1997.1179. J Mol Biol. 1997. PMID: 9325096
-
Atomic structure of the major capsid protein of rotavirus: implications for the architecture of the virion.EMBO J. 2001 Apr 2;20(7):1485-97. doi: 10.1093/emboj/20.7.1485. EMBO J. 2001. PMID: 11285213 Free PMC article.
-
Rotavirus Replication: Gaps of Knowledge on Virus Entry and Morphogenesis.Tohoku J Exp Med. 2019 Aug;248(4):285-296. doi: 10.1620/tjem.248.285. Tohoku J Exp Med. 2019. PMID: 31447474 Review.
-
Structural studies on orbivirus proteins and particles.Curr Top Microbiol Immunol. 2006;309:221-44. doi: 10.1007/3-540-30773-7_8. Curr Top Microbiol Immunol. 2006. PMID: 16909901 Review.
Cited by
-
On the effect of thermodynamic equilibrium on the assembly efficiency of complex multi-layered virus-like particles (VLP): the case of rotavirus VLP.PLoS Comput Biol. 2012;8(2):e1002367. doi: 10.1371/journal.pcbi.1002367. Epub 2012 Feb 16. PLoS Comput Biol. 2012. PMID: 22359487 Free PMC article.
-
Fabs enable single particle cryoEM studies of small proteins.Structure. 2012 Apr 4;20(4):582-92. doi: 10.1016/j.str.2012.02.017. Epub 2012 Apr 3. Structure. 2012. PMID: 22483106 Free PMC article.
-
Interactions among capsid proteins orchestrate rotavirus particle functions.Curr Opin Virol. 2012 Aug;2(4):373-9. doi: 10.1016/j.coviro.2012.04.005. Epub 2012 May 16. Curr Opin Virol. 2012. PMID: 22595300 Free PMC article. Review.
-
Generation of single-round infectious rotavirus with a mutation in the intermediate capsid protein VP6.J Virol. 2024 Jul 23;98(7):e0076224. doi: 10.1128/jvi.00762-24. Epub 2024 Jun 5. J Virol. 2024. PMID: 38837379 Free PMC article.
-
Single-Particle Cryo-EM at Crystallographic Resolution.Cell. 2015 Apr 23;161(3):450-457. doi: 10.1016/j.cell.2015.03.049. Cell. 2015. PMID: 25910205 Free PMC article. Review.
References
-
- Estes MK, Kapikian AZ. Rotaviruses. In: Howley DMKPM, editor. Fields Virology. 5th ed. Philadelphia: Lippincott, Williams & Wilkins; 2007. pp. 1918–1974.
-
- Adams WR, Kraft LM. Epizootic diarrhea of infant mice: Indentification of the etiologic agent. Science. 1963;141:359–360. - PubMed
-
- Altenburg BC, Graham DY, Estes MK. Ultrastructural study of rotavirus replication in cultured cells. J Gen Virol. 1980;46:75–85. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

