GNL3L stabilizes the TRF1 complex and promotes mitotic transition
- PMID: 19487455
- PMCID: PMC2711588
- DOI: 10.1083/jcb.200812121
GNL3L stabilizes the TRF1 complex and promotes mitotic transition
Abstract
Telomeric repeat binding factor 1 (TRF1) is a component of the multiprotein complex "shelterin," which organizes the telomere into a high-order structure. TRF1 knockout embryos suffer from severe growth defects without apparent telomere dysfunction, suggesting an obligatory role for TRF1 in cell cycle control. To date, the mechanism regulating the mitotic increase in TRF1 protein expression and its function in mitosis remains unclear. Here, we identify guanine nucleotide-binding protein-like 3 (GNL3L), a GTP-binding protein most similar to nucleostemin, as a novel TRF1-interacting protein in vivo. GNL3L binds TRF1 in the nucleoplasm and is capable of promoting the homodimerization and telomeric association of TRF1, preventing promyelocytic leukemia body recruitment of telomere-bound TRF1, and stabilizing TRF1 protein by inhibiting its ubiquitylation and binding to FBX4, an E3 ubiquitin ligase for TRF1. Most importantly, the TRF1 protein-stabilizing activity of GNL3L mediates the mitotic increase of TRF1 protein and promotes the metaphase-to-anaphase transition. This work reveals novel aspects of TRF1 modulation by GNL3L.
Figures
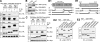

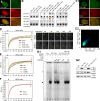
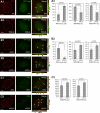


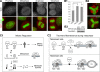
Similar articles
-
Nucleostemin inhibits TRF1 dimerization and shortens its dynamic association with the telomere.J Cell Sci. 2011 Nov 1;124(Pt 21):3706-14. doi: 10.1242/jcs.089672. Epub 2011 Nov 1. J Cell Sci. 2011. PMID: 22045740 Free PMC article.
-
Nucleolar modulation of TRF1: a dynamic way to regulate telomere and cell cycle by nucleostemin and GNL3L.Cell Cycle. 2009 Sep 15;8(18):2912-6. doi: 10.4161/cc.8.18.9543. Epub 2009 Sep 16. Cell Cycle. 2009. PMID: 19713769 Free PMC article. Review.
-
The F-box protein FBX4 targets PIN2/TRF1 for ubiquitin-mediated degradation and regulates telomere maintenance.J Biol Chem. 2006 Jan 13;281(2):759-68. doi: 10.1074/jbc.M509855200. Epub 2005 Nov 7. J Biol Chem. 2006. PMID: 16275645
-
The splicing factor U2AF65 stabilizes TRF1 protein by inhibiting its ubiquitin-dependent proteolysis.Biochem Biophys Res Commun. 2014 Jan 17;443(3):1124-30. doi: 10.1016/j.bbrc.2013.12.118. Epub 2013 Dec 31. Biochem Biophys Res Commun. 2014. PMID: 24389012
-
Role of Pin2/TRF1 in telomere maintenance and cell cycle control.J Cell Biochem. 2003 May 1;89(1):19-37. doi: 10.1002/jcb.10496. J Cell Biochem. 2003. PMID: 12682905 Review.
Cited by
-
New prospects for targeting telomerase beyond the telomere.Nat Rev Cancer. 2016 Aug;16(8):508-24. doi: 10.1038/nrc.2016.55. Epub 2016 Jun 24. Nat Rev Cancer. 2016. PMID: 27339602 Review.
-
Regulation of ribosome biogenesis by nucleostemin 3 promotes local and systemic growth in Drosophila.Genetics. 2013 May;194(1):101-15. doi: 10.1534/genetics.112.149104. Epub 2013 Feb 22. Genetics. 2013. PMID: 23436180 Free PMC article.
-
GNL3L Is a Nucleo-Cytoplasmic Shuttling Protein: Role in Cell Cycle Regulation.PLoS One. 2015 Aug 14;10(8):e0135845. doi: 10.1371/journal.pone.0135845. eCollection 2015. PLoS One. 2015. PMID: 26274615 Free PMC article.
-
The human telomeric proteome during telomere replication.Nucleic Acids Res. 2021 Dec 2;49(21):12119-12135. doi: 10.1093/nar/gkab1015. Nucleic Acids Res. 2021. PMID: 34747482 Free PMC article.
-
Arabidopsis NUCLEOSTEMIN-LIKE 1 (NSN1) regulates cell cycling potentially by cooperating with nucleosome assembly protein AtNAP1;1.BMC Plant Biol. 2018 Jun 1;18(1):99. doi: 10.1186/s12870-018-1289-2. BMC Plant Biol. 2018. PMID: 29859040 Free PMC article.
References
-
- Bilaud T., Brun C., Ancelin K., Koering C.E., Laroche T., Gilson E. 1997. Telomeric localization of TRF2, a novel human telobox protein.Nat. Genet. 17:236–239 - PubMed
-
- Broccoli D., Smogorzewska A., Chong L., de Lange T. 1997. Human telomeres contain two distinct Myb-related proteins, TRF1 and TRF2.Nat. Genet. 17:231–235 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

