Striatal overexpression of DeltaJunD resets L-DOPA-induced dyskinesia in a primate model of Parkinson disease
- PMID: 19481198
- PMCID: PMC2825375
- DOI: 10.1016/j.biopsych.2009.04.005
Striatal overexpression of DeltaJunD resets L-DOPA-induced dyskinesia in a primate model of Parkinson disease
Abstract
Background: Involuntary movements, or dyskinesia, represent a debilitating complication of dopamine replacement therapy for Parkinson disease (PD). The transcription factor DeltaFosB accumulates in the denervated striatum and dimerizes primarily with JunD upon repeated L-3,4-dihydroxyphenylalanine (L-DOPA) administration. Previous studies in rodents have shown that striatal DeltaFosB levels accurately predict dyskinesia severity and indicate that this transcription factor may play a causal role in the dyskinesia sensitization process.
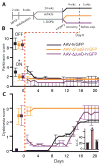
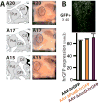
Methods: We asked whether the correlation previously established in rodents extends to the best nonhuman primate model of PD, the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned macaque. We used western blotting and quantitative polymerase chain reaction (PCR) to compare DeltaFosB protein and messenger RNA (mRNA) levels across two subpopulations of macaques with differential dyskinesia severity. Second, we tested the causal implication of DeltaFosB in this primate model. Serotype 2 adeno-associated virus (AAV2) vectors were used to overexpress, within the motor striatum, either DeltaFosB or DeltaJunD, a truncated variant of JunD lacking a transactivation domain and therefore acting as a dominant negative inhibitor of DeltaFosB.
Results: A linear relationship was observed between endogenous striatal levels of DeltaFosB and the severity of dyskinesia in Parkinsonian macaques treated with L-DOPA. Viral overexpression of DeltaFosB did not alter dyskinesia severity in animals previously rendered dyskinetic, whereas the overexpression of DeltaJunD dramatically dropped the severity of this side effect of L-DOPA without altering the antiparkinsonian activity of the treatment.
Conclusions: These results establish a mechanism of dyskinesia induction and maintenance by L-DOPA and validate a strategy, with strong translational potential, to deprime the L-DOPA-treated brain.
Figures

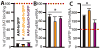
Similar articles
-
Role of striatal ΔFosB in l-Dopa-induced dyskinesias of parkinsonian nonhuman primates.Proc Natl Acad Sci U S A. 2019 Sep 10;116(37):18664-18672. doi: 10.1073/pnas.1907810116. Epub 2019 Aug 27. Proc Natl Acad Sci U S A. 2019. PMID: 31455727 Free PMC article.
-
Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of deltaFosB-like protein(s) in both the rodent and primate striatum.Eur J Neurosci. 1996 Feb;8(2):365-81. doi: 10.1111/j.1460-9568.1996.tb01220.x. Eur J Neurosci. 1996. PMID: 8714707
-
Effect of non-dopaminergic drug treatment on Levodopa induced dyskinesias in MPTP monkeys: common implication of striatal neuropeptides.Neuropharmacology. 2010 Jan;58(1):286-96. doi: 10.1016/j.neuropharm.2009.06.030. Epub 2009 Jul 2. Neuropharmacology. 2010. PMID: 19576910
-
Animal models of l-dopa-induced dyskinesia in Parkinson's disease.Mov Disord. 2018 Jul;33(6):889-899. doi: 10.1002/mds.27337. Epub 2018 Feb 28. Mov Disord. 2018. PMID: 29488257 Review.
-
Animal models of Parkinson's disease and L-dopa induced dyskinesia: how close are we to the clinic?Psychopharmacology (Berl). 2008 Aug;199(3):303-12. doi: 10.1007/s00213-007-0931-8. Epub 2007 Sep 25. Psychopharmacology (Berl). 2008. PMID: 17899020 Review.
Cited by
-
The Signaling and Pharmacology of the Dopamine D1 Receptor.Front Cell Neurosci. 2022 Jan 17;15:806618. doi: 10.3389/fncel.2021.806618. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35110997 Free PMC article.
-
Integrated transcriptome expression profiling reveals a novel lncRNA associated with L-DOPA-induced dyskinesia in a rat model of Parkinson's disease.Aging (Albany NY). 2020 Jan 10;12(1):718-739. doi: 10.18632/aging.102652. Epub 2020 Jan 10. Aging (Albany NY). 2020. PMID: 31929116 Free PMC article.
-
Small molecule screening identifies regulators of the transcription factor ΔFosB.ACS Chem Neurosci. 2012 Jul 18;3(7):546-56. doi: 10.1021/cn3000235. Epub 2012 Mar 29. ACS Chem Neurosci. 2012. PMID: 22860224 Free PMC article.
-
A Pharmacogenetic Discovery: Cystamine Protects Against Haloperidol-Induced Toxicity and Ischemic Brain Injury.Genetics. 2016 May;203(1):599-609. doi: 10.1534/genetics.115.184648. Epub 2016 Mar 18. Genetics. 2016. PMID: 26993135 Free PMC article.
-
Discovery of phenanthridine analogues as novel chemical probes disrupting the binding of DNA to ΔFosB homodimers and ΔFosB/JunD heterodimers.Bioorg Med Chem Lett. 2020 Aug 15;30(16):127300. doi: 10.1016/j.bmcl.2020.127300. Epub 2020 Jun 6. Bioorg Med Chem Lett. 2020. PMID: 32631520 Free PMC article.
References
-
- Nicholas AP, Lubin FD, Hallett PJ, Vattem P, Ravenscroft P, Bezard E, et al. Striatal histone modifications in models of levodopa-induced dyskinesia. J Neurochem. 2008;106:486–494. - PubMed
-
- Cenci MA. Transcription factors involved in the pathogenesis of L-DOPA-induced dyskinesia in a rat model of Parkinson’s disease. Amino Acids. 2002;23:105–109. - PubMed
-
- Doucet JP, Nakabeppu Y, Bedard PJ, Hope BT, Nestler EJ, Jasmin BJ, et al. Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of deltaFosB-like protein(s) in both the rodent and primate striatum. Eur J Neurosci. 1996;8:365–381. - PubMed
-
- Vallone D, Pellecchia MT, Morelli M, Verde P, DiChiara G, Barone P. Behavioural sensitization in 6-hydroxydopamine-lesioned rats is related to compositional changes of the AP-1 transcription factor: evidence for induction of FosB- and JunD-related proteins. Brain Res Mol Brain Res. 1997;52:307–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

