In vivo and in vitro effects of two novel gamma-actin (ACTG1) mutations that cause DFNA20/26 hearing impairment
- PMID: 19477959
- PMCID: PMC2714729
- DOI: 10.1093/hmg/ddp249
In vivo and in vitro effects of two novel gamma-actin (ACTG1) mutations that cause DFNA20/26 hearing impairment
Abstract
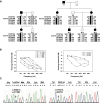
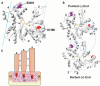
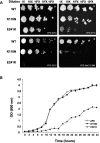
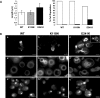
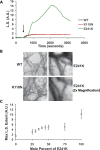
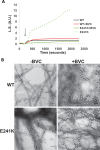
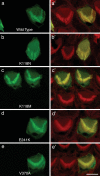
Here we report the functional assessment of two novel deafness-associated gamma-actin mutants, K118N and E241K, in a spectrum of different situations with increasing biological complexity by combining biochemical and cell biological analysis in yeast and mammalian cells. Our in vivo experiments showed that while the K118N had a very mild effect on yeast behaviour, the phenotype caused by the E241K mutation was very severe and characterized by a highly compromised ability to grow on glycerol as a carbon source, an aberrant multi-vacuolar pattern and the deposition of thick F-actin bundles randomly in the cell. The latter feature is consistent with the highly unusual spontaneous tendency of the E241K mutant to form bundles in vitro, although this propensity to bundle was neutralized by tropomyosin and the E241K filament bundles were hypersensitive to severing in the presence of cofilin. In transiently transfected NIH3T3 cells both mutant actins were normally incorporated into cytoskeleton structures, although cytoplasmic aggregates were also observed indicating an element of abnormality caused by the mutations in vivo. Interestingly, gene-gun mediated expression of these mutants in cochlear hair cells results in no gross alteration in cytoskeletal structures or the morphology of stereocilia. Our results provide a more complete picture of the biological consequences of deafness-associated gamma-actin mutants and support the hypothesis that the post-lingual and progressive nature of the DFNA20/26 hearing loss is the result of a progressive deterioration of the hair cell cytoskeleton over time.
Figures


Similar articles
-
Allele-specific effects of human deafness gamma-actin mutations (DFNA20/26) on the actin/cofilin interaction.J Biol Chem. 2009 Jul 3;284(27):18260-9. doi: 10.1074/jbc.M109.015818. Epub 2009 May 6. J Biol Chem. 2009. PMID: 19419963 Free PMC article.
-
A novel missense mutation in ACTG1 causes dominant deafness in a Norwegian DFNA20/26 family, but ACTG1 mutations are not frequent among families with hereditary hearing impairment.Eur J Hum Genet. 2006 Oct;14(10):1097-105. doi: 10.1038/sj.ejhg.5201670. Epub 2006 Jun 14. Eur J Hum Genet. 2006. PMID: 16773128
-
Novel ACTG1 mutations in patients identified by massively parallel DNA sequencing cause progressive hearing loss.Sci Rep. 2020 Apr 27;10(1):7056. doi: 10.1038/s41598-020-63690-5. Sci Rep. 2020. PMID: 32341388 Free PMC article.
-
Actin in hair cells and hearing loss.Hear Res. 2012 Jun;288(1-2):89-99. doi: 10.1016/j.heares.2011.12.003. Epub 2011 Dec 13. Hear Res. 2012. PMID: 22200607 Free PMC article. Review.
-
Stereocilia morphogenesis and maintenance through regulation of actin stability.Semin Cell Dev Biol. 2017 May;65:88-95. doi: 10.1016/j.semcdb.2016.08.017. Epub 2016 Aug 23. Semin Cell Dev Biol. 2017. PMID: 27565685 Free PMC article. Review.
Cited by
-
Amino acid 118 in the Deafness Causing (DFNA20/26) ACTG1 gene is a Mutational Hot Spot.Gene Rep. 2018 Jun;11:264-269. doi: 10.1016/j.genrep.2018.04.011. Epub 2018 Apr 28. Gene Rep. 2018. PMID: 30599039 Free PMC article.
-
Structural polymorphism in F-actin.Nat Struct Mol Biol. 2010 Nov;17(11):1318-23. doi: 10.1038/nsmb.1930. Epub 2010 Oct 10. Nat Struct Mol Biol. 2010. PMID: 20935633 Free PMC article.
-
De Novo ACTG1 Variant Expands the Phenotype and Genotype of Partial Deafness and Baraitser-Winter Syndrome.Int J Mol Sci. 2022 Jan 8;23(2):692. doi: 10.3390/ijms23020692. Int J Mol Sci. 2022. PMID: 35054877 Free PMC article.
-
Recent advances in genetic etiology of non-syndromic deafness in children.Front Neurosci. 2023 Oct 19;17:1282663. doi: 10.3389/fnins.2023.1282663. eCollection 2023. Front Neurosci. 2023. PMID: 37928735 Free PMC article. Review.
-
Conformational dynamics of actin: effectors and implications for biological function.Cytoskeleton (Hoboken). 2010 Oct;67(10):609-29. doi: 10.1002/cm.20473. Cytoskeleton (Hoboken). 2010. PMID: 20672362 Free PMC article. Review.
References
-
- Hudspeth A.J. How the ear's works work. Nature. 1989;341:397–404. - PubMed
-
- Tilney L.G., Egelman E.H., DeRosier D.J., Saunder J.C. Actin filaments, stereocilia, and hair cells of the bird cochlea. II. Packing of actin filaments in the stereocilia and in the cuticular plate and what happens to the organization when the stereocilia are bent. J. Cell Biol. 1983;96:822–834. - PMC - PubMed
-
- Kabsch W., Mannherz H.G., Suck D., Pai E.F., Holmes K.C. Atomic structure of the actin: DNase I complex. Nature. 1990;347:37–44. - PubMed
-
- De La Cruz E.M. Cofilin binding to muscle and non-muscle actin filaments: isoform-dependent cooperative interactions. J. Mol. Biol. 2005;346:557–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

