Generalized X-ray and neutron crystallographic analysis: more accurate and complete structures for biological macromolecules
- PMID: 19465771
- PMCID: PMC2685734
- DOI: 10.1107/S0907444909011548
Generalized X-ray and neutron crystallographic analysis: more accurate and complete structures for biological macromolecules
Abstract
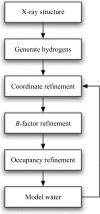
X-ray and neutron crystallographic techniques provide complementary information on the structure and function of biological macromolecules. X-ray and neutron (XN) crystallographic data have been combined in a joint structure-refinement procedure that has been developed using recent advances in modern computational methodologies, including cross-validated maximum-likelihood target functions with gradient-based optimization and simulated annealing. The XN approach for complete (including hydrogen) macromolecular structure analysis provides more accurate and complete structures, as demonstrated for diisopropyl fluorophosphatase, photoactive yellow protein and human aldose reductase. Furthermore, this method has several practical advantages, including the easier determination of the orientation of water molecules, hydroxyl groups and some amino-acid side chains.
Figures
Similar articles
-
Rapid determination of hydrogen positions and protonation states of diisopropyl fluorophosphatase by joint neutron and X-ray diffraction refinement.Proc Natl Acad Sci U S A. 2009 Jan 20;106(3):713-8. doi: 10.1073/pnas.0807842106. Epub 2009 Jan 9. Proc Natl Acad Sci U S A. 2009. PMID: 19136630 Free PMC article.
-
X-ray structure of perdeuterated diisopropyl fluorophosphatase (DFPase): perdeuteration of proteins for neutron diffraction.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010 Apr 1;66(Pt 4):379-85. doi: 10.1107/S1744309110004318. Epub 2010 Mar 26. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010. PMID: 20383004 Free PMC article.
-
Neutron and X-ray structural studies of short hydrogen bonds in photoactive yellow protein (PYP).Acta Crystallogr D Biol Crystallogr. 2007 Nov;63(Pt 11):1178-84. doi: 10.1107/S0907444907047646. Epub 2007 Oct 17. Acta Crystallogr D Biol Crystallogr. 2007. PMID: 18007033 Free PMC article.
-
Neutron Crystallography for the Study of Hydrogen Bonds in Macromolecules.Molecules. 2017 Apr 7;22(4):596. doi: 10.3390/molecules22040596. Molecules. 2017. PMID: 28387738 Free PMC article. Review.
-
Recent developments for the efficient crystallographic refinement of macromolecular structures.Curr Opin Struct Biol. 1998 Oct;8(5):606-11. doi: 10.1016/s0959-440x(98)80152-8. Curr Opin Struct Biol. 1998. PMID: 9818265 Review.
Cited by
-
Universality of critical active site glutamate as an acid-base catalyst in serine hydroxymethyltransferase function.Chem Sci. 2024 Jul 3;15(32):12827-12844. doi: 10.1039/d4sc03187c. eCollection 2024 Aug 14. Chem Sci. 2024. PMID: 39148791 Free PMC article.
-
Neutron diffraction from a microgravity-grown crystal reveals the active site hydrogens of the internal aldimine form of tryptophan synthase.Cell Rep Phys Sci. 2024 Feb 21;5(2):101827. doi: 10.1016/j.xcrp.2024.101827. Epub 2024 Feb 12. Cell Rep Phys Sci. 2024. PMID: 38645802 Free PMC article.
-
Diverse array of neutralizing antibodies elicited upon Spike Ferritin Nanoparticle vaccination in rhesus macaques.Nat Commun. 2024 Jan 3;15(1):200. doi: 10.1038/s41467-023-44265-0. Nat Commun. 2024. PMID: 38172512 Free PMC article.
-
Improved joint X-ray and neutron refinement procedure in Phenix.Acta Crystallogr D Struct Biol. 2023 Dec 1;79(Pt 12):1079-1093. doi: 10.1107/S2059798323008914. Epub 2023 Nov 9. Acta Crystallogr D Struct Biol. 2023. PMID: 37942718 Free PMC article.
-
Neutron crystallographic refinement with REFMAC5 from the CCP4 suite.Acta Crystallogr D Struct Biol. 2023 Dec 1;79(Pt 12):1056-1070. doi: 10.1107/S2059798323008793. Epub 2023 Nov 3. Acta Crystallogr D Struct Biol. 2023. PMID: 37921806 Free PMC article.
References
-
- Adams, P. D., Grosse-Kunstleve, R. W., Hung, L.-W., Ioerger, T. R., McCoy, A. J., Moriarty, N. W., Read, R. J., Sacchettini, J. C., Sauter, N. K. & Terwilliger, T. C. (2002). Acta Cryst. D58, 1948–1954. - PubMed
-
- Afonine, P. V., Grosse-Kunstleve, R. W. & Adams, P. D. (2005). CCP4 Newsl. 42, contribution 8.
-
- Bennett, B. C., Gardberg, A. S., Blair, M. D. & Dealwis, C. G. (2008). Acta Cryst. D64, 764–783. - PubMed
-
- Berman, H. M. et al. (2002). Acta Cryst. D58, 899–907. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

