Regulation of cancer invasiveness by the physical extracellular matrix environment
- PMID: 19458499
- PMCID: PMC2712813
- DOI: 10.4161/cam.3.3.8888
Regulation of cancer invasiveness by the physical extracellular matrix environment
Abstract
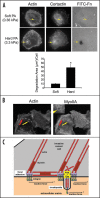
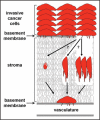
Long-term clinical outcomes are dependent on whether carcinoma cells leave the primary tumor site and invade through adjacent tissue. Recent evidence links tissue rigidity to alterations in cancer cell phenotype and tumor progression. We found that rigid extracellular matrix (ECM) substrates promote invasiveness of tumor cells via increased activity of invadopodia, subcellular protrusions with associated ECM-degrading proteinases. Although the subcellular mechanism by which substrate rigidity promotes invadopodia function remains to be determined, force sensing does appear to occur through myosin-based contractility and the mechanosensing proteins FAK and p130(Cas). In addition to rigidity, a number of ECM characteristics may regulate the ability of cells to invade through tissues, including matrix density and crosslinking. 3-D biological hydrogels based on type I collagen and reconstituted basement membrane are commonly used to study invasive behavior; however, these models lack some of the tissue-specific properties found in vivo. Thus, new in vitro organotypic and synthetic polymer ECM substrate models will be useful to either mimic the properties of specific ECM microenvironments encountered by invading cancer cells or to manipulate ECM substrate properties and independently test the role of rigidity, integrin ligands, pore size and proteolytic activity in cancer invasion of various tissues.
Figures
Similar articles
-
Extracellular matrix rigidity promotes invadopodia activity.Curr Biol. 2008 Sep 9;18(17):1295-1299. doi: 10.1016/j.cub.2008.07.090. Epub 2008 Aug 21. Curr Biol. 2008. PMID: 18718759 Free PMC article.
-
Matrix rigidity differentially regulates invadopodia activity through ROCK1 and ROCK2.Biomaterials. 2016 Apr;84:119-129. doi: 10.1016/j.biomaterials.2016.01.028. Epub 2016 Jan 15. Biomaterials. 2016. PMID: 26826790 Free PMC article.
-
The matrix environmental and cell mechanical properties regulate cell migration and contribute to the invasive phenotype of cancer cells.Rep Prog Phys. 2019 Jun;82(6):064602. doi: 10.1088/1361-6633/ab1628. Epub 2019 Apr 4. Rep Prog Phys. 2019. PMID: 30947151 Review.
-
Cellular traction stresses mediate extracellular matrix degradation by invadopodia.Acta Biomater. 2014 May;10(5):1886-96. doi: 10.1016/j.actbio.2013.12.058. Epub 2014 Jan 8. Acta Biomater. 2014. PMID: 24412623 Free PMC article.
-
Regulation of invadopodia by mechanical signaling.Exp Cell Res. 2016 Apr 10;343(1):89-95. doi: 10.1016/j.yexcr.2015.10.038. Epub 2015 Nov 4. Exp Cell Res. 2016. PMID: 26546985 Free PMC article. Review.
Cited by
-
Expression of human papillomavirus oncoproteins E6 and E7 inhibits invadopodia activity but promotes cell migration in HPV-positive head and neck squamous cell carcinoma cells.Cancer Rep (Hoboken). 2018 Oct;1(3):e1125. doi: 10.1002/cnr2.1125. Epub 2018 Jul 27. Cancer Rep (Hoboken). 2018. PMID: 32721084 Free PMC article.
-
Direct measurement of vertical forces shows correlation between mechanical activity and proteolytic ability of invadopodia.Sci Adv. 2020 Mar 11;6(11):eaax6912. doi: 10.1126/sciadv.aax6912. eCollection 2020 Mar. Sci Adv. 2020. PMID: 32195338 Free PMC article.
-
Cortactin function in invadopodia.Small GTPases. 2020 Jul;11(4):256-270. doi: 10.1080/21541248.2017.1405773. Epub 2017 Dec 31. Small GTPases. 2020. PMID: 29172953 Free PMC article. Review.
-
Hic-5 promotes invadopodia formation and invasion during TGF-β-induced epithelial-mesenchymal transition.J Cell Biol. 2012 Apr 30;197(3):421-37. doi: 10.1083/jcb.201108143. Epub 2012 Apr 23. J Cell Biol. 2012. PMID: 22529104 Free PMC article.
-
Geometric constraints of endothelial cell migration on electrospun fibres.Sci Rep. 2018 Apr 23;8(1):6386. doi: 10.1038/s41598-018-24667-7. Sci Rep. 2018. PMID: 29686428 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
