Focal adhesion kinase signaling regulates the expression of caveolin 3 and beta1 integrin, genes essential for normal myoblast fusion
- PMID: 19458188
- PMCID: PMC2710835
- DOI: 10.1091/mbc.e09-02-0175
Focal adhesion kinase signaling regulates the expression of caveolin 3 and beta1 integrin, genes essential for normal myoblast fusion
Abstract
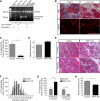
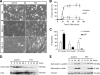
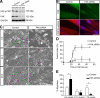
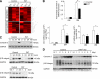
An essential phase of skeletal myogenesis is the fusion of mononucleated myoblasts to form multinucleated myotubes. Many cell adhesion proteins, including integrins, have been shown to be important for myoblast fusion in vertebrates, but the mechanisms by which these proteins regulate cell fusion remain mostly unknown. Here, we focused on the role of focal adhesion kinase (FAK), an important nonreceptor protein tyrosine kinase involved in integrin signaling, as a potential mediator by which integrins may regulate myoblast fusion. To test this hypothesis in vivo, we generated mice in which the Fak gene was disrupted specifically in muscle stem cells ("satellite cells") and we found that this resulted in impaired myotube formation during muscle regeneration after injury. To examine the role of FAK in the fusion of myogenic cells, we examined the expression of FAK and the effects of FAK deletion on the differentiation of myoblasts in vitro. Differentiation of mouse primary myoblasts was accompanied by a rapid and transient increase of phosphorylated FAK. To investigate the requirement of FAK in myoblast fusion, we used two loss-of-function approaches (a dominant-negative inhibitor of FAK and FAK small interfering RNA [siRNA]). Inhibition of FAK resulted in markedly impaired fusion but did not inhibit other biochemical measures of myogenic differentiation, suggesting a specific role of FAK in the morphological changes of cell fusion as part of the differentiation program. To examine the mechanisms by which FAK may be regulating fusion, we used microarray analysis to identify the genes that failed to be normally regulated in cells that were fusion defective due to FAK inhibition. Several genes that have been implicated in myoblast fusion were aberrantly regulated during differentiation when FAK was inhibited. Intriguingly, the normal increases in the transcript of caveolin 3 as well as an integrin subunit, the beta1D isoform, were suppressed by FAK inhibition. We confirmed this also at the protein level and show that direct inhibition of beta1D subunit expression by siRNA inhibited myotube formation with a prominent effect on secondary fusion. These data suggest that FAK regulation of profusion genes, including caveolin 3 and the beta1D integrin subunit, is essential for morphological muscle differentiation.
Figures


Similar articles
-
PKCθ signaling is required for myoblast fusion by regulating the expression of caveolin-3 and β1D integrin upstream focal adhesion kinase.Mol Biol Cell. 2011 Apr 15;22(8):1409-19. doi: 10.1091/mbc.E10-10-0821. Epub 2011 Feb 23. Mol Biol Cell. 2011. PMID: 21346196 Free PMC article.
-
Quantitative changes in integrin and focal adhesion signaling regulate myoblast cell cycle withdrawal.J Cell Biol. 1999 Mar 22;144(6):1295-309. doi: 10.1083/jcb.144.6.1295. J Cell Biol. 1999. PMID: 10087271 Free PMC article.
-
TMEM182 interacts with integrin beta 1 and regulates myoblast differentiation and muscle regeneration.J Cachexia Sarcopenia Muscle. 2021 Dec;12(6):1704-1723. doi: 10.1002/jcsm.12767. Epub 2021 Aug 23. J Cachexia Sarcopenia Muscle. 2021. PMID: 34427057 Free PMC article.
-
Mechanisms regulating myoblast fusion: A multilevel interplay.Semin Cell Dev Biol. 2020 Aug;104:81-92. doi: 10.1016/j.semcdb.2020.02.004. Epub 2020 Feb 13. Semin Cell Dev Biol. 2020. PMID: 32063453 Review.
-
Regulation of promyogenic signal transduction by cell-cell contact and adhesion.Exp Cell Res. 2010 Nov 1;316(18):3042-9. doi: 10.1016/j.yexcr.2010.05.008. Epub 2010 May 21. Exp Cell Res. 2010. PMID: 20471976 Free PMC article. Review.
Cited by
-
Signaling mechanisms in mammalian myoblast fusion.Sci Signal. 2013 Apr 23;6(272):re2. doi: 10.1126/scisignal.2003832. Sci Signal. 2013. PMID: 23612709 Free PMC article. Review.
-
Protein tyrosine phosphatase-like A regulates myoblast proliferation and differentiation through MyoG and the cell cycling signaling pathway.Mol Cell Biol. 2012 Jan;32(2):297-308. doi: 10.1128/MCB.05484-11. Epub 2011 Nov 21. Mol Cell Biol. 2012. PMID: 22106411 Free PMC article.
-
Adhesion proteins--an impact on skeletal myoblast differentiation.PLoS One. 2013 May 6;8(5):e61760. doi: 10.1371/journal.pone.0061760. Print 2013. PLoS One. 2013. PMID: 23671573 Free PMC article.
-
Shisa2 regulates the fusion of muscle progenitors.Stem Cell Res. 2018 Aug;31:31-41. doi: 10.1016/j.scr.2018.07.004. Epub 2018 Jul 6. Stem Cell Res. 2018. PMID: 30007221 Free PMC article.
-
Talin 1 and 2 are required for myoblast fusion, sarcomere assembly and the maintenance of myotendinous junctions.Development. 2009 Nov;136(21):3597-606. doi: 10.1242/dev.035857. Epub 2009 Sep 30. Development. 2009. PMID: 19793892 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

