Neurotrophin-3 targets the translational initiation machinery in oligodendrocytes
- PMID: 19455580
- PMCID: PMC4300950
- DOI: 10.1002/glia.20888
Neurotrophin-3 targets the translational initiation machinery in oligodendrocytes
Abstract
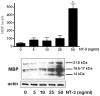
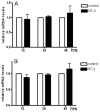
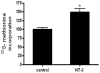
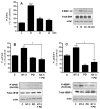
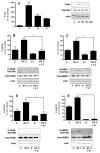
Neurotrophin-3 (NT-3) regulates oligodendrocyte (OLG) differentiation by mechanisms that remain poorly understood. Exposure of OLGs to NT-3 induces a significant increase in the levels of myelin basic protein (MBP). However, we found that this stimulation occurs in the absence of measurable effects on MBP gene promoter activation or mRNA expression, suggesting that NT-3 upregulates MBP protein expression by a posttranscriptional mechanism. Furthermore, NT-3 also causes an increase in the levels of myelin-associated glycoprotein (MAG) and myelin OLG glycoprotein (MOG), raising the possibility of a more general effect on myelin protein synthesis. Surprisingly, (35)S-methionine incorporation into total OLG proteins demonstrated a 50% increase in labeling following only a brief, 15-min treatment with NT-3. Such a remarkably fast response is unlikely due to transcriptional activation, reinforcing the possibility that NT-3 may play a crucial role in regulating protein expression by a posttranscriptional mechanism. In support of this idea, we found that NT-3 stimulates the phosphorylation of essential regulators of the initiation machinery, eukaryotic initiation factor 4E (eIF4E), and its inhibitory binding partner 4E binding protein 1 (4EBP1), two crucial players in controlling cap-dependent protein synthesis. This stimulation involves the activation of pathways mediated by ERK1/2 and PI3K/mTOR, implicating these two kinase systems as modulators of protein synthesis in developing OLGs. Altogether, these observations show for the first time that NT-3 has the capacity of targeting the translational machinery and suggest a potential stimulatory effect of this neurotrophin on myelination by direct action on protein translation in the OLGs.
Figures
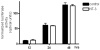
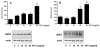

Similar articles
-
Erk1/2 but not PI3K pathway is required for neurotrophin 3-induced oligodendrocyte differentiation of post-natal neural stem cells.J Neurochem. 2004 Sep;90(6):1339-47. doi: 10.1111/j.1471-4159.2004.02594.x. J Neurochem. 2004. PMID: 15341518
-
NT-3 weakly stimulates proliferation of adult rat O1(-)O4(+) oligodendrocyte-lineage cells and increases oligodendrocyte myelination in vitro.J Neurosci Res. 2000 Nov 1;62(3):329-35. doi: 10.1002/1097-4547(20001101)62:3<329::AID-JNR2>3.0.CO;2-C. J Neurosci Res. 2000. PMID: 11054801
-
Neurotrophin-3 and a CREB-mediated signaling pathway regulate Bcl-2 expression in oligodendrocyte progenitor cells.J Neurochem. 2004 May;89(4):951-61. doi: 10.1111/j.1471-4159.2004.02365.x. J Neurochem. 2004. PMID: 15140194
-
Neu differentiation factor regulates tau protein and mRNA in cultured neonatal oligodendrocytes.Glia. 2001 Aug;35(2):147-55. doi: 10.1002/glia.1079. Glia. 2001. PMID: 11460270
-
Possible role of CREB in the stimulation of oligodendrocyte precursor cell proliferation by neurotrophin-3.J Neurochem. 2000 Apr;74(4):1409-17. doi: 10.1046/j.1471-4159.2000.0741409.x. J Neurochem. 2000. PMID: 10737596
Cited by
-
Endogenous and exogenous opioid effects on oligodendrocyte biology and developmental brain myelination.Neurotoxicol Teratol. 2021 Jul-Aug;86:107002. doi: 10.1016/j.ntt.2021.107002. Epub 2021 Jun 12. Neurotoxicol Teratol. 2021. PMID: 34126203 Free PMC article. Review.
-
Conditional knockout of TOG results in CNS hypomyelination.Glia. 2017 Mar;65(3):489-501. doi: 10.1002/glia.23106. Epub 2017 Jan 7. Glia. 2017. PMID: 28063167 Free PMC article.
-
The PI3K-AKT-mTOR signaling pathway mediates the cytoskeletal remodeling and epithelial-mesenchymal transition in bladder outlet obstruction.Heliyon. 2023 Nov 2;9(11):e21281. doi: 10.1016/j.heliyon.2023.e21281. eCollection 2023 Nov. Heliyon. 2023. PMID: 38027933 Free PMC article.
-
PharmGKB summary: methylphenidate pathway, pharmacokinetics/pharmacodynamics.Pharmacogenet Genomics. 2019 Aug;29(6):136-154. doi: 10.1097/FPC.0000000000000376. Pharmacogenet Genomics. 2019. PMID: 30950912 Free PMC article. Review. No abstract available.
-
Neuroglialpharmacology: myelination as a shared mechanism of action of psychotropic treatments.Neuropharmacology. 2012 Jun;62(7):2137-53. doi: 10.1016/j.neuropharm.2012.01.015. Epub 2012 Jan 28. Neuropharmacology. 2012. PMID: 22306524 Free PMC article. Review.
References
-
- Armstrong RC. Isolation and characterization of immature oligodendrocyte lineage cells. Methods. 1998;16(3):282–92. - PubMed
-
- Banko JL, Klann E. Cap-dependent translation initiation and memory. Prog Brain Res. 2008;169:59–80. - PubMed
-
- Baron W, Metz B, Bansal R, Hoekstra D, de Vries H. PDGF and FGF-2 signaling in oligodendrocyte progenitor cells: regulation of proliferation and differentiation by multiple intracellular signaling pathways. Mol Cell Neurosci. 2000;15(3):314–29. - PubMed
-
- Barres BA, Raff MC. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12(5):935–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

