Aberrant cell cycle progression and endoreplication in regenerating livers of mice that lack a single E-type cyclin
- PMID: 19445941
- PMCID: PMC2730664
- DOI: 10.1053/j.gastro.2009.05.003
Aberrant cell cycle progression and endoreplication in regenerating livers of mice that lack a single E-type cyclin
Abstract
Background & aims: E-cyclins control the transition of quiescent cells into the cell cycle. Two E-cyclins, CcnE1 and CcnE2, have been described, but their specific contributions to cell cycle reentry in vivo are poorly understood. Liver regeneration following partial hepatectomy is an excellent in vivo model for the study of cell cycle reentry of quiescent cells. We investigated the relevance of E-cyclins in directing resting hepatocytes into the cell cycle after partial hepatectomy using CcnE1 and CcnE2 knockout mice.
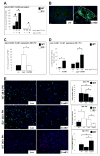
Methods: Partial hepatectomy (70%) was performed in CcnE1 (E1(-/-)) and CcnE2 (E2(-/-)) knockout and wild-type mice. Liver regeneration was monitored by cell cycle markers for G(1)/S phase, S phase, and M phase as well as by determining the liver/body weight ratio after partial hepatectomy. Ploidy of hepatocytes was determined by fluorescence-activated cell sorting and fluorescent in situ hybridization.
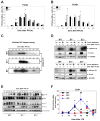
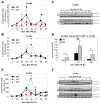
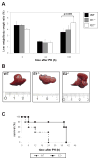
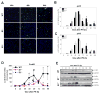
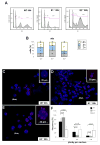
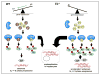
Results: CcnE1 deletion resulted in normal liver regeneration with a slight delay of the G(1)/S-phase transition and a defect in endoreplication of otherwise polyploid hepatocytes. Surprisingly, E2(-/-) mice displayed accelerated and sustained DNA synthesis after partial hepatectomy, excessive endoreplication in hepatocytes, and a liver mass that was 45% greater than that of wild-type mice after termination of the regeneration process. CcnE2 depletion induced overexpression of CcnE1 and prolonged cdk2 kinase activity after partial hepatectomy.
Conclusions: CcnE2 has an unexpected role in repressing CcnE1; the phenotype of E2(-/-) mice appears to result from CcnE1 overexpression and cdk2 hyperactivation. CcnE1 and CcnE2 therefore have nonredundant functions for S-phase entry and endoreplication during liver regeneration.
Conflict of interest statement
The authors report no financial conflicts of interest
Figures
References
-
- Kaldis P, Aleem E. Cell cycle sibling rivalry: Cdc2 vs. Cdk2. Cell Cycle. 2005;4:1491–4. - PubMed
-
- Geng Y, Yu Q, Sicinska E, et al. Cyclin E ablation in the mouse. Cell. 2003;114:431–43. - PubMed
-
- Koff A, Cross F, Fisher A, et al. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991;66:1217–28. - PubMed
-
- Lauper N, Beck AR, Cariou S, et al. Cyclin E2: a novel CDK2 partner in the late G1 and S phases of the mammalian cell cycle. Oncogene. 1998;17:2637–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

