Modulation of p53, c-fos, RARE, cyclin A, and cyclin D1 expression in human leukemia (HL-60) cells exposed to arsenic trioxide
- PMID: 19444595
- PMCID: PMC2855208
- DOI: 10.1007/s11010-009-0160-z
Modulation of p53, c-fos, RARE, cyclin A, and cyclin D1 expression in human leukemia (HL-60) cells exposed to arsenic trioxide
Abstract
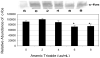
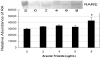
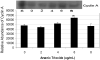
Arsenic trioxide (As(2)O(3)) has recently been successfully used to treat all trans retinoic acid (ATRA) resistant relapsing acute promyelocytic leukemia. However, its molecular mechanisms of action are poorly understood. In the present study, we used the human leukemia (HL-60) cell line as a test model to study the cellular and molecular mechanisms of anti-cancer properties of As(2)O(3). We hypothesized that As(2)O(3)-induced expression of stress genes and related proteins may play a role in the cellular and molecular events leading to cell cycle modulation in leukemic cells. To test this hypothesis, we performed Western blot analysis to assess the expression of specific cellular response proteins including p53, c-fos, RARE, Cyclin A, and Cyclin D1. Densitometric analysis was performed to determine the relative abundance of these proteins. Western Blot and densitometric analyses demonstrated a strong dose-response relationship with regard to p53 and RARE expression within the dose-range of 0-8 microg/ml. Expression of c-fos was slightly up-regulated at 2 microg/ml, and down-regulated within the dose-range of 4-8 microg/ml. A statistically significant down-regulation of this protein was detected at the 6 and 8 microg/ml dose levels. No statistically significant differences (p > 0.05) in Cyclin D1 expression was found between As(2)O(3)-treated cells and the control. Cyclin A expression in As(2)O(3)-treated HL-60 cells was up-regulated at 6 microg/ml, suggesting that it is required for S phase and passage through G(2) phase in cell cycle progression. Taken together, these results indicate that As(2)O(3) has the potential to induce cell cycle arrest through activation of the 53-kDa tumor suppressor protein and repression of the c-fos transcription factor. Up-regulation of RARE by As(2)O(3) indicates that its cytotoxicity may be mediated through interaction/binding with the retinoic acid receptor, and subsequent inhibition of growth and differentiation.
Figures
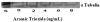
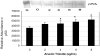
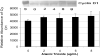
Similar articles
-
Arsenic trioxide-induced transcriptional activation of stress genes and expression of related proteins in human liver carcinoma cells (HepG2).Cell Mol Biol (Noisy-le-grand). 2003 Nov;49(7):1071-9. Cell Mol Biol (Noisy-le-grand). 2003. PMID: 14682389
-
Arsenic trioxide and the growth of human T-cell leukemia virus type I infected T-cell lines.Leuk Lymphoma. 2000 May;37(5-6):649-55. doi: 10.3109/10428190009058521. Leuk Lymphoma. 2000. PMID: 11042529
-
Arsenic trioxide induces apoptosis in pancreatic cancer cells via changes in cell cycle, caspase activation, and GADD expression.Pancreas. 2003 Aug;27(2):174-9. doi: 10.1097/00006676-200308000-00011. Pancreas. 2003. PMID: 12883267
-
Arsenic-induced apoptosis in malignant cells in vitro.Leuk Lymphoma. 2000 Mar;37(1-2):53-63. doi: 10.3109/10428190009057628. Leuk Lymphoma. 2000. PMID: 10721769 Review.
-
Arsenic trioxide therapy for relapsed acute promyelocytic leukemia: an useful salvage therapy.Leuk Lymphoma. 2000 Jul;38(3-4):283-93. doi: 10.3109/10428190009087019. Leuk Lymphoma. 2000. PMID: 10830735 Review.
Cited by
-
Therapeutic Potential of Arsenic Trioxide (ATO) in Treatment of Hepatocellular Carcinoma: Role of Oxidative Stress in ATO-Induced Apoptosis.Ann Clin Pathol. 2017;5(1):1101. Epub 2017 Jan 4. Ann Clin Pathol. 2017. PMID: 29214213 Free PMC article.
-
Multifactorial Modes of Action of Arsenic Trioxide in Cancer Cells as Analyzed by Classical and Network Pharmacology.Front Pharmacol. 2018 Feb 27;9:143. doi: 10.3389/fphar.2018.00143. eCollection 2018. Front Pharmacol. 2018. PMID: 29535630 Free PMC article.
-
Basic mechanisms of arsenic trioxide (ATO)-induced apoptosis in human leukemia (HL-60) cells.J Hematol Oncol. 2010 Aug 26;3:28. doi: 10.1186/1756-8722-3-28. J Hematol Oncol. 2010. PMID: 20796291 Free PMC article.
-
Arsenite causes down-regulation of Akt and c-Fos, cell cycle dysfunction and apoptosis in glutathione-deficient cells.J Cell Biochem. 2010 May 15;110(2):363-71. doi: 10.1002/jcb.22548. J Cell Biochem. 2010. PMID: 20336670 Free PMC article.
-
Heavy metal toxicity and the environment.Exp Suppl. 2012;101:133-64. doi: 10.1007/978-3-7643-8340-4_6. Exp Suppl. 2012. PMID: 22945569 Free PMC article. Review.
References
-
- Chen GQ, Zhu J, Shi XG, Ni HJ, Zhong HJ, Si GY, Jin XL, Tang W, Li XS, Xong SM, Shen ZX, Sun GL, Ma J, Zhang P, Zhang TD, Gazin C, Naoe T, Chen SJ, Wang ZY, Chen Z. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: arsenic trioxide induces NB 4 cell apoptosis with down-regulation of bcl-2 expression and modulation of PML-RARα/PML proteins. Blood. 1996;88:1052–1061. - PubMed
-
- Rego EM, He LZ, Warrell RP, Jr, Wang ZG, Pandolfi PP. Retinoic acid (RA) and As2O3 treatment in transgenic models of acute promyelocytic leukemia (APL) unravel the distinct nature of the leukemiamogenic process induced by the PML-RARapha and PLZF-RARalpha oncoproteins. Proc Natl Acad Sci USA. 2000;97:10173–10178. - PMC - PubMed
-
- Bode A, Dong Z. Apoptosis induction by arsenic: mechanisms of action and possible clinical applications for treating therapy-resistant cancers. J Drug Resist Updat. 2000;3:21–29. - PubMed
-
- Bode AM, Dong Z. The paradox of arsenic: molecular mechanisms of cell transformation and chemotherapeutic effects. Crit Rev Oncol/Hematol. 2002;42:5–24. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

