Characterization of a highly conserved domain within the severe acute respiratory syndrome coronavirus spike protein S2 domain with characteristics of a viral fusion peptide
- PMID: 19439480
- PMCID: PMC2708636
- DOI: 10.1128/JVI.00079-09
Characterization of a highly conserved domain within the severe acute respiratory syndrome coronavirus spike protein S2 domain with characteristics of a viral fusion peptide
Abstract
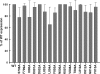
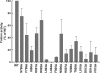
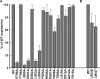
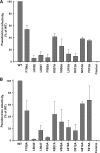
Many viral fusion proteins are primed by proteolytic cleavage near their fusion peptides. While the coronavirus (CoV) spike (S) protein is known to be cleaved at the S1/S2 boundary, this cleavage site is not closely linked to a fusion peptide. However, a second cleavage site has been identified in the severe acute respiratory syndrome CoV (SARS-CoV) S2 domain (R797). Here, we investigated whether this internal cleavage of S2 exposes a viral fusion peptide. We show that the residues immediately C-terminal to the SARS-CoV S2 cleavage site SFIEDLLFNKVTLADAGF are very highly conserved across all CoVs. Mutagenesis studies of these residues in SARS-CoV S, followed by cell-cell fusion and pseudotyped virion infectivity assays, showed a critical role for residues L803, L804, and F805 in membrane fusion. Mutation of the most N-terminal residue (S798) had little or no effect on membrane fusion. Biochemical analyses of synthetic peptides corresponding to the proposed S2 fusion peptide also showed an important role for this region in membrane fusion and indicated the presence of alpha-helical structure. We propose that proteolytic cleavage within S2 exposes a novel internal fusion peptide for SARS-CoV S, which may be conserved across the Coronaviridae.
Figures
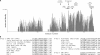



Similar articles
-
SARS-coronavirus spike S2 domain flanked by cysteine residues C822 and C833 is important for activation of membrane fusion.Virology. 2009 Oct 25;393(2):265-71. doi: 10.1016/j.virol.2009.07.038. Epub 2009 Aug 29. Virology. 2009. PMID: 19717178 Free PMC article.
-
Identification and characterization of the putative fusion peptide of the severe acute respiratory syndrome-associated coronavirus spike protein.J Virol. 2005 Jun;79(11):7195-206. doi: 10.1128/JVI.79.11.7195-7206.2005. J Virol. 2005. PMID: 15890958 Free PMC article.
-
Elastase-mediated activation of the severe acute respiratory syndrome coronavirus spike protein at discrete sites within the S2 domain.J Biol Chem. 2010 Jul 23;285(30):22758-63. doi: 10.1074/jbc.M110.103275. Epub 2010 May 27. J Biol Chem. 2010. PMID: 20507992 Free PMC article.
-
The spike protein of SARS-CoV--a target for vaccine and therapeutic development.Nat Rev Microbiol. 2009 Mar;7(3):226-36. doi: 10.1038/nrmicro2090. Epub 2009 Feb 9. Nat Rev Microbiol. 2009. PMID: 19198616 Free PMC article. Review.
-
The SARS-CoV S glycoprotein.Cell Mol Life Sci. 2004 Oct;61(19-20):2428-30. doi: 10.1007/s00018-004-4257-y. Cell Mol Life Sci. 2004. PMID: 15526150 Free PMC article. Review.
Cited by
-
Identification of the Fusion Peptide-Containing Region in Betacoronavirus Spike Glycoproteins.J Virol. 2016 May 27;90(12):5586-5600. doi: 10.1128/JVI.00015-16. Print 2016 Jun 15. J Virol. 2016. PMID: 27030273 Free PMC article.
-
Mechanisms of coronavirus cell entry mediated by the viral spike protein.Viruses. 2012 Jun;4(6):1011-33. doi: 10.3390/v4061011. Epub 2012 Jun 20. Viruses. 2012. PMID: 22816037 Free PMC article. Review.
-
A forward genetic strategy reveals destabilizing mutations in the Ebolavirus glycoprotein that alter its protease dependence during cell entry.J Virol. 2010 Jan;84(1):163-75. doi: 10.1128/JVI.01832-09. J Virol. 2010. PMID: 19846533 Free PMC article.
-
An in silico approach unveils the potential of antiviral compounds in preclinical and clinical trials as SARS-CoV-2 omicron inhibitors.Saudi J Biol Sci. 2022 Jun;29(6):103297. doi: 10.1016/j.sjbs.2022.103297. Epub 2022 Apr 22. Saudi J Biol Sci. 2022. PMID: 35475118 Free PMC article.
-
PEDV: Insights and Advances into Types, Function, Structure, and Receptor Recognition.Viruses. 2022 Aug 9;14(8):1744. doi: 10.3390/v14081744. Viruses. 2022. PMID: 36016366 Free PMC article. Review.
References
-
- Bosch, B. J., and P. J. Rottier. 2008. Nidovirus Entry into Cells, p. 157-178. In S. Perlman, T. Gallagher, and E. J. Snijder (ed.), Nidoviruses. ASM Press, Washington, DC.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

