Combination use of immune complexes and a Ca2(+) channel blocker azelnidipine enhances interleukin-12 p40 secretion without T helper type 17 cytokine secretion in human monocyte-derived dendritic cells
- PMID: 19438591
- PMCID: PMC2691967
- DOI: 10.1111/j.1365-2249.2009.03911.x
Combination use of immune complexes and a Ca2(+) channel blocker azelnidipine enhances interleukin-12 p40 secretion without T helper type 17 cytokine secretion in human monocyte-derived dendritic cells
Abstract
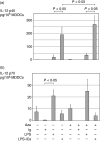
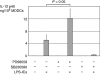
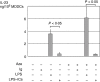
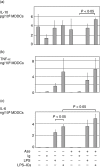
Immune complexes (ICs) improve the capacity of priming specific CD8(+) cytotoxic T cell responses of dendritic cells (DCs). ICs induce phosphorylation of mitogen-activated protein kinases (MAPK) and calcium influx, although the precise regulating mechanism still remains unclear. In the present study, we investigated the effect of a Ca2(+) channel blocker on the phosphorylation of p38 MAPK and extracellular signal-regulated kinase (ERK) in immature monocyte-derived DCs stimulated with lipopolysaccharide (LPS) or LPS-ICs, and the production of interleukin (IL)-12 family members (p40, p70, IL-23), T helper type 17 (Th17) cytokines (IL-6 and IL-23), tumour necrosis factor (TNF)-alpha and IL-10 were also investigated. In comparison with LPS stimulation, LPS-ICs stimulation enhanced p38 MAPK phosphorylation significantly, which was associated with an increase in IL-12 p40 monomer/homodimer secretion. LPS-ICs also enhanced TNF-alpha and IL-6 secretion, but suppressed IL-23 secretion. The use of azelnidipine (Aze), a long-acting L-type Ca2(+) channel blocker with a high lipid solubility, suppressed p38 MAPK phosphorylation stimulated with LPS or LPS-ICs, but surprisingly enhanced IL-12 p40 monomer/homodimer secretion stimulated with LPS-ICs. This IL-12 p40 secretion-enhancing effect was not accompanied by IL-10 or IL-23 production, but was associated with ERK phosphorylation. The use of Aze did not affect IL-12 p70 production. These results suggest that the use of Aze enhances ICs-mediated IL-12 p40 secretion without additional IL-23 secretion. Therefore, the use of Aze and ICs could be a new therapeutic approach to immunomolecular therapy, as it does not cause Th17 differentiation which induces autoimmunity or reduces anti-tumour immunity.
Figures
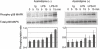
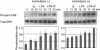
Similar articles
-
Selective synergy in anti-inflammatory cytokine production upon cooperated signaling via TLR4 and TLR2 in murine conventional dendritic cells.Mol Immunol. 2008 May;45(10):2734-42. doi: 10.1016/j.molimm.2008.02.010. Epub 2008 Mar 26. Mol Immunol. 2008. PMID: 18372043
-
Sphingosine-1-phosphate differently regulates the cytokine production of IL-12, IL-23 and IL-27 in activated murine bone marrow derived dendritic cells.Mol Immunol. 2014 May;59(1):10-8. doi: 10.1016/j.molimm.2013.11.015. Epub 2014 Jan 14. Mol Immunol. 2014. PMID: 24434636
-
RIP2 mediates LPS-induced p38 and IkappaBalpha signaling including IL-12 p40 expression in human monocyte-derived dendritic cells.Eur J Immunol. 2007 Aug;37(8):2317-25. doi: 10.1002/eji.200636388. Eur J Immunol. 2007. PMID: 17578844
-
Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans.Immunol Rev. 2008 Dec;226:112-31. doi: 10.1111/j.1600-065X.2008.00700.x. Immunol Rev. 2008. PMID: 19161420 Free PMC article. Review.
-
Interleukin-12: Structure, Function, and Its Impact in Colorectal Cancer.J Interferon Cytokine Res. 2024 Apr;44(4):158-169. doi: 10.1089/jir.2023.0190. Epub 2024 Mar 18. J Interferon Cytokine Res. 2024. PMID: 38498032 Review.
Cited by
-
Verification of the effects of calcium channel blockers on the immune microenvironment of breast cancer.BMC Cancer. 2019 Jun 24;19(1):615. doi: 10.1186/s12885-019-5828-5. BMC Cancer. 2019. PMID: 31234828 Free PMC article.
-
Verapamil results in increased blood levels of oncolytic adenovirus in treatment of patients with advanced cancer.Mol Ther. 2012 Jan;20(1):221-9. doi: 10.1038/mt.2011.230. Epub 2011 Nov 1. Mol Ther. 2012. PMID: 22044933 Free PMC article.
-
Repositioning Azelnidipine as a Dual Inhibitor Targeting CD47/SIRPα and TIGIT/PVR Pathways for Cancer Immuno-Therapy.Biomolecules. 2021 May 10;11(5):706. doi: 10.3390/biom11050706. Biomolecules. 2021. PMID: 34068552 Free PMC article.
References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
-
- Macatonia SE, Hosken NA, Litton M, et al. Dendritic cells produce IL-12 and direct the development of Th1 cells from naive CD4+ T cells. J Immunol. 1995;154:5071–9. - PubMed
-
- Trinchieri G, Scott P. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res Immunol. 1995;146:423–31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

