Dose escalation methods in phase I cancer clinical trials
- PMID: 19436029
- PMCID: PMC2684552
- DOI: 10.1093/jnci/djp079
Dose escalation methods in phase I cancer clinical trials
Abstract
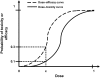
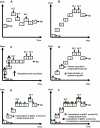
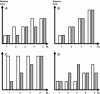
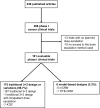
Phase I clinical trials are an essential step in the development of anticancer drugs. The main goal of these studies is to establish the recommended dose and/or schedule of new drugs or drug combinations for phase II trials. The guiding principle for dose escalation in phase I trials is to avoid exposing too many patients to subtherapeutic doses while preserving safety and maintaining rapid accrual. Here we review dose escalation methods for phase I trials, including the rule-based and model-based dose escalation methods that have been developed to evaluate new anticancer agents. Toxicity has traditionally been the primary endpoint for phase I trials involving cytotoxic agents. However, with the emergence of molecularly targeted anticancer agents, potential alternative endpoints to delineate optimal biological activity, such as plasma drug concentration and target inhibition in tumor or surrogate tissues, have been proposed along with new trial designs. We also describe specific methods for drug combinations as well as methods that use a time-to-event endpoint or both toxicity and efficacy as endpoints. Finally, we present the advantages and drawbacks of the various dose escalation methods and discuss specific applications of the methods in developmental oncotherapeutics.
Figures
Comment in
-
Re: Dose escalation methods in phase I cancer clinical trials.J Natl Cancer Inst. 2009 Dec 16;101(24):1732-3; author reply 1733-5. doi: 10.1093/jnci/djp400. J Natl Cancer Inst. 2009. PMID: 19893006 No abstract available.
Similar articles
-
The performance of model-based versus rule-based phase I clinical trials in oncology : A quantitative comparison of the performance of model-based versus rule-based phase I trials with molecularly targeted anticancer drugs over the last 2 years.J Pharmacokinet Pharmacodyn. 2016 Jun;43(3):235-42. doi: 10.1007/s10928-016-9466-0. Epub 2016 Mar 10. J Pharmacokinet Pharmacodyn. 2016. PMID: 26960536 Review.
-
NCI-RTOG translational program strategic guidelines for the early-stage development of radiosensitizers.J Natl Cancer Inst. 2013 Jan 2;105(1):11-24. doi: 10.1093/jnci/djs472. Epub 2012 Dec 10. J Natl Cancer Inst. 2013. PMID: 23231975 Free PMC article.
-
Revisiting the definition of dose-limiting toxicities in paediatric oncology phase I clinical trials: An analysis from the Innovative Therapies for Children with Cancer Consortium.Eur J Cancer. 2017 Nov;86:275-284. doi: 10.1016/j.ejca.2017.09.015. Epub 2017 Oct 19. Eur J Cancer. 2017. PMID: 29055843
-
Re: Dose escalation methods in phase I cancer clinical trials.J Natl Cancer Inst. 2009 Dec 16;101(24):1732-3; author reply 1733-5. doi: 10.1093/jnci/djp400. J Natl Cancer Inst. 2009. PMID: 19893006 No abstract available.
-
The changing landscape of phase I trials in oncology.Nat Rev Clin Oncol. 2016 Feb;13(2):106-17. doi: 10.1038/nrclinonc.2015.194. Epub 2015 Nov 10. Nat Rev Clin Oncol. 2016. PMID: 26552953 Review.
Cited by
-
Phase 1b study of the oral gemcitabine 'Pro-drug' LY2334737 in combination with capecitabine in patients with advanced solid tumors.Invest New Drugs. 2015 Apr;33(2):432-9. doi: 10.1007/s10637-015-0207-9. Epub 2015 Feb 3. Invest New Drugs. 2015. PMID: 25640850 Clinical Trial.
-
A robust two-stage design identifying the optimal biological dose for phase I/II clinical trials.Stat Med. 2017 Jan 15;36(1):27-42. doi: 10.1002/sim.7082. Epub 2016 Aug 18. Stat Med. 2017. PMID: 27538818 Free PMC article.
-
Permitting patients to pay for participation in clinical trials: the advent of the P4 trial.Med Health Care Philos. 2017 Jun;20(2):219-227. doi: 10.1007/s11019-016-9741-2. Med Health Care Philos. 2017. PMID: 27757784 Free PMC article.
-
Preclinical discovery of candidate genes to guide pharmacogenetics during phase I development: the example of the novel anticancer agent ABT-751.Pharmacogenet Genomics. 2013 Jul;23(7):374-81. doi: 10.1097/FPC.0b013e3283623e81. Pharmacogenet Genomics. 2013. PMID: 23670235 Free PMC article. Clinical Trial.
-
Improving access to novel agents for childhood leukemia.Cancer. 2015 Jun 15;121(12):1927-36. doi: 10.1002/cncr.29267. Epub 2015 Feb 11. Cancer. 2015. PMID: 25678105 Free PMC article. Review.
References
-
- Korn EL, Arbuck SG, Pluda JM, Simon R, Kaplan RS, Christian MC. Clinical trial designs for cytostatic agents: are new approaches needed? J Clin Oncol. 2001;19(1):265–272. - PubMed
-
- Parulekar WR, Eisenhauer EA. Phase I trial design for solid tumor studies of targeted, non-cytotoxic agents: theory and practice. J Natl Cancer Inst. 2004;96(13):990–997. - PubMed
-
- Sleijfer S, Wiemer E. Dose selection in phase I studies: why we should always go for the top. J Clin Oncol. 2008;26(10):1576–1578. - PubMed
-
- Cannistra SA. Challenges and pitfalls of combining targeted agents in phase I studies. J Clin Oncol. 2008;26(22):3665–3667. - PubMed
-
- Rogatko A, Schoeneck D, Jonas W, Tighiouart M, Khuri FR, Porter A. Translation of innovative designs into phase I trials. J Clin Oncol. 2007;25(31):4982–4986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

