Novel role of FATP1 in mitochondrial fatty acid oxidation in skeletal muscle cells
- PMID: 19429947
- PMCID: PMC2724792
- DOI: 10.1194/jlr.M800535-JLR200
Novel role of FATP1 in mitochondrial fatty acid oxidation in skeletal muscle cells
Abstract
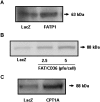
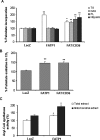
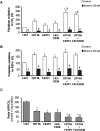
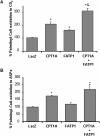
Carnitine palmitoyltransferase 1 (CPT1) catalyzes the first step in long-chain fatty acid import into mitochondria, and it is believed to be rate limiting for beta-oxidation of fatty acids. However, in muscle, other proteins may collaborate with CPT1. Fatty acid translocase/CD36 (FAT/CD36) may interact with CPT1 and contribute to fatty acid import into mitochondria in muscle. Here, we demonstrate that another membrane-bound fatty acid binding protein, fatty acid transport protein 1 (FATP1), collaborates with CPT1 for fatty acid import into mitochondria. Overexpression of FATP1 using adenovirus in L6E9 myotubes increased both fatty acid oxidation and palmitate esterification into triacylglycerides. Moreover, immunocytochemistry assays in transfected L6E9 myotubes showed that FATP1 was present in mitochondria and coimmunoprecipitated with CPT1 in L6E9 myotubes and rat skeletal muscle in vivo. The cooverexpression of FATP1 and CPT1 also enhanced mitochondrial fatty acid oxidation, similar to the cooverexpression of FAT/CD36 and CPT1. However, etomoxir, an irreversible inhibitor of CPT1, blocked all these effects. These data reveal that FATP1, like FAT/CD36, is associated with mitochondria and has a role in mitochondrial oxidation of fatty acids.
Figures
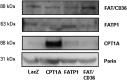
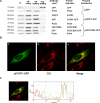

Similar articles
-
HIV-protease inhibitors suppress skeletal muscle fatty acid oxidation by reducing CD36 and CPT1 fatty acid transporters.Biochim Biophys Acta. 2010 May;1801(5):559-66. doi: 10.1016/j.bbalip.2010.01.007. Epub 2010 Feb 1. Biochim Biophys Acta. 2010. PMID: 20117238 Free PMC article.
-
Fatty acid transport protein 1 (FATP1) localizes in mitochondria in mouse skeletal muscle and regulates lipid and ketone body disposal.PLoS One. 2014 May 23;9(5):e98109. doi: 10.1371/journal.pone.0098109. eCollection 2014. PLoS One. 2014. PMID: 24858472 Free PMC article.
-
Greater transport efficiencies of the membrane fatty acid transporters FAT/CD36 and FATP4 compared with FABPpm and FATP1 and differential effects on fatty acid esterification and oxidation in rat skeletal muscle.J Biol Chem. 2009 Jun 12;284(24):16522-16530. doi: 10.1074/jbc.M109.004788. Epub 2009 Apr 20. J Biol Chem. 2009. PMID: 19380575 Free PMC article.
-
Contribution of FAT/CD36 to the regulation of skeletal muscle fatty acid oxidation: an overview.Acta Physiol (Oxf). 2008 Dec;194(4):293-309. doi: 10.1111/j.1748-1716.2008.01878.x. Epub 2008 Jul 9. Acta Physiol (Oxf). 2008. PMID: 18510711 Review.
-
Molecular Regulation of Fatty Acid Oxidation in Skeletal Muscle during Aerobic Exercise.Trends Endocrinol Metab. 2018 Jan;29(1):18-30. doi: 10.1016/j.tem.2017.10.011. Epub 2017 Dec 5. Trends Endocrinol Metab. 2018. PMID: 29221849 Review.
Cited by
-
Genetic Variation of Fatty Acid Oxidation and Obesity, A Literature Review.Int J Biomed Sci. 2016 Mar;12(1):1-8. Int J Biomed Sci. 2016. PMID: 27127449 Free PMC article. Review.
-
Fatty acid metabolism in aggressive B-cell lymphoma is inhibited by tetraspanin CD37.Nat Commun. 2022 Sep 13;13(1):5371. doi: 10.1038/s41467-022-33138-7. Nat Commun. 2022. PMID: 36100608 Free PMC article.
-
Activation of AMPKα2 Is Not Required for Mitochondrial FAT/CD36 Accumulation during Exercise.PLoS One. 2015 May 12;10(5):e0126122. doi: 10.1371/journal.pone.0126122. eCollection 2015. PLoS One. 2015. PMID: 25965390 Free PMC article.
-
α-MSH and Foxc2 promote fatty acid oxidation through C/EBPβ negative transcription in mice adipose tissue.Sci Rep. 2016 Nov 7;6:36661. doi: 10.1038/srep36661. Sci Rep. 2016. PMID: 27819350 Free PMC article.
-
Metabolic heterogeneity of idiopathic pulmonary fibrosis: a metabolomic study.BMJ Open Respir Res. 2017 Jun 5;4(1):e000183. doi: 10.1136/bmjresp-2017-000183. eCollection 2017. BMJ Open Respir Res. 2017. PMID: 28883924 Free PMC article.
References
-
- Bonen A., Chabowski A., Luiken J. J., Glatz J. F. 2007. Is membrane transport of FFA mediated by lipid, protein, or both? Mechanisms and regulation of protein-mediated cellular fatty acid uptake: molecular, biochemical, and physiological evidence. Physiology (Bethesda). 22: 15–29. - PubMed
-
- McGarry J. D., Brown N. F. 1997. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 244: 1–14. - PubMed
-
- Esser V., Britton C. H., Weis B. C., Foster D. W., McGarry J. D. 1993. Cloning, sequencing, and expression of a cDNA encoding rat liver carnitine palmitoyltransferase I. Direct evidence that a single polypeptide is involved in inhibitor interaction and catalytic function. J. Biol. Chem. 268: 5817–5822. - PubMed
-
- Yamazaki N., Shinohara Y., Shima A., Terada H. 1995. High expression of a novel carnitine palmitoyltransferase I like protein in rat brown adipose tissue and heart: isolation and characterization of its cDNA clone. FEBS Lett. 363: 41–45. - PubMed
-
- Price N., van der Leij F., Jackson V., Corstorphine C., Thomson R., Sorensen A., Zammit V. 2002. A novel brain-expressed protein related to carnitine palmitoyltransferase I. Genomics. 80: 433–442. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

