Role of cell signaling in poxvirus-mediated foreign gene expression in mammalian cells
- PMID: 19428911
- PMCID: PMC3189381
- DOI: 10.1016/j.vaccine.2009.02.103
Role of cell signaling in poxvirus-mediated foreign gene expression in mammalian cells
Abstract
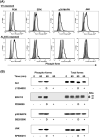
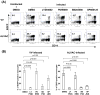
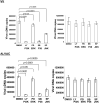
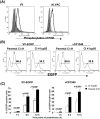
Poxviruses have been extensively used as a promising vehicle to efficiently deliver a variety of antigens in mammalian hosts to induce immune responses against infectious diseases and cancer. Using recombinant vaccinia virus (VV) and canarypox virus (ALVAC) expressing enhanced green fluorescent protein (EGFP) or multiple HIV-1 gene products, we studied the role of four cellular signaling pathways, the phosphoinositide-3-OH kinase (PI3K), extracellular signal-regulated kinase (ERK), p38 mitogen-activated protein kinase (p38 MAPK), and c-Jun N-terminal kinase (JNK), in poxvirus-mediated foreign gene expression in mammalian cells. In nonpermissive infection (human monocytes), activation of PI3K, ERK, p38 MAPK, and JNK was observed in both VV and ALVAC and blocking PI3K, p38 MAKP, and JNK pathways with their specific inhibitors significantly reduced viral and vaccine antigen gene expression. Whereas, blocking the ERK pathway had no significant effect. Among these cellular signaling pathways studied, PI3K was the most critical pathway involved in gene expression by VV- or ALVAC-infected monocytes. The important role of PI3K in poxvirus-mediated gene expression was further confirmed in mouse epidermal cells stably transfected with dominant-negative PI3K mutant, as poxvirus-mediated targeted gene expression was significantly decreased in these cells when compared with their parental cells. Signaling pathway activation influenced gene expression at the mRNA level rather than virus binding. In permissive mammalian cells, however, VV DNA copies were also significantly decreased in the absence of normal function of the PI3K pathway. Poxvirus-triggered activation of PI3K pathway could be completely abolished by atazanavir, a new generation of antiretroviral protease inhibitors (PIs). As a consequence, ALVAC-mediated EGFP or HIV-1 gag gene expression in infected primary human monocytes was significantly reduced in the presence of atazanavir. These findings implicate that antiretroviral therapy (ART), also known as highly active antiretroviral therapy (HAART), may negatively impact the efficacy of live poxvirus vector-based vaccines and should be carefully considered when administering such live vaccines to individuals on ART.
Figures



Similar articles
-
Gene profiling analysis of ALVAC infected human monocyte derived dendritic cells.Vaccine. 2008 Sep 15;26(39):5004-13. doi: 10.1016/j.vaccine.2008.07.050. Epub 2008 Aug 6. Vaccine. 2008. PMID: 18691624 Free PMC article.
-
Comparative analysis of tropism between canarypox (ALVAC) and vaccinia viruses reveals a more restricted and preferential tropism of ALVAC for human cells of the monocytic lineage.Vaccine. 2006 Sep 29;24(40-41):6376-91. doi: 10.1016/j.vaccine.2006.06.011. Epub 2006 Jun 30. Vaccine. 2006. PMID: 16859816
-
The canarypox virus vector ALVAC induces distinct cytokine responses compared to the vaccinia virus-based vectors MVA and NYVAC in rhesus monkeys.J Virol. 2014 Feb;88(3):1809-14. doi: 10.1128/JVI.02386-13. Epub 2013 Nov 20. J Virol. 2014. PMID: 24257612 Free PMC article.
-
Prospects and limitations of recombinant poxviruses for prostate cancer immunotherapy.Curr Opin Mol Ther. 1999 Aug;1(4):471-9. Curr Opin Mol Ther. 1999. PMID: 11713762 Review.
-
Poxvirus-based vectors as vaccine candidates.Crit Rev Immunol. 1990;10(1):13-30. Crit Rev Immunol. 1990. PMID: 2407263 Review.
Cited by
-
Multiple phosphatidylinositol 3-kinases regulate vaccinia virus morphogenesis.PLoS One. 2010 May 28;5(5):e10884. doi: 10.1371/journal.pone.0010884. PLoS One. 2010. PMID: 20526370 Free PMC article.
-
Primary human leukocyte subsets differentially express vaccinia virus receptors enriched in lipid rafts.J Virol. 2013 Aug;87(16):9301-12. doi: 10.1128/JVI.01545-13. Epub 2013 Jun 19. J Virol. 2013. PMID: 23785200 Free PMC article.
-
JNK2 modulates the CD1d-dependent and -independent activation of iNKT cells.Eur J Immunol. 2019 Feb;49(2):255-265. doi: 10.1002/eji.201847755. Epub 2018 Dec 3. Eur J Immunol. 2019. PMID: 30467836 Free PMC article.
-
A loss of function analysis of host factors influencing Vaccinia virus replication by RNA interference.PLoS One. 2014 Jun 5;9(6):e98431. doi: 10.1371/journal.pone.0098431. eCollection 2014. PLoS One. 2014. PMID: 24901222 Free PMC article.
-
Primary human macrophages serve as vehicles for vaccinia virus replication and dissemination.J Virol. 2014 Jun;88(12):6819-31. doi: 10.1128/JVI.03726-13. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696488 Free PMC article.
References
-
- Moss B. Vaccinia and other poxvirus expression vectors. Curr Opin Biotechnol. 1992 Oct;3(5):518–22. - PubMed
-
- Moss B, Fuerst TR, Flexner C, Hugin A. Roles of vaccinia virus in the development of new vaccines. Vaccine. 1988 Apr;6(2):161–3. - PubMed
-
- Moss B, Flexner C. Vaccinia virus expression vectors. Annu Rev Immunol. 1987;5:305–24. - PubMed
-
- Poulet H, Minke J, Pardo MC, Juillard V, Nordgren B, Audonnet JC. Development and registration of recombinant veterinary vaccines. The example of the canarypox vector platform. Vaccine. 2007 Jul 26;25(30):5606–12. - PubMed
-
- Perkus ME, Tartaglia J, Paoletti E. Poxvirus-based vaccine candidates for cancer, AIDS, and other infectious diseases. J Leukoc Biol. 1995 Jul;58(1):1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

