Functional assessment of isolated mitochondria in vitro
- PMID: 19426878
- PMCID: PMC2782617
- DOI: 10.1016/S0076-6879(09)05020-4
Functional assessment of isolated mitochondria in vitro
Abstract
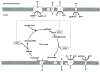
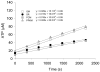
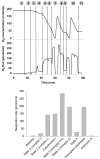
Mitochondria play a pivotal role in cellular function, not only as a major site of ATP production, but also by regulating energy expenditure, apoptosis signaling, and production of reactive oxygen species. Altered mitochondrial function is reported to be a key underlying mechanism of many pathological states and in the aging process. Functional measurements of intact mitochondria isolated from fresh tissue provides distinct information regarding the function of these organelles that complements conventional mitochondrial assays using previously frozen tissue as well as in vivo assessment using techniques such as magnetic resonance and near-infrared spectroscopy. This chapter describes the process by which mitochondria are isolated from small amounts of human skeletal muscle obtained by needle biopsy and two approaches used to assess mitochondrial oxidative capacity and other key components of mitochondrial physiology. We first describe a bioluminescent approach for measuring the rates of mitochondrial ATP production. Firefly luciferase catalyzes a light-emitting reaction whereby the substrate luciferin is oxidized in an ATP-dependent manner. A luminometer is used to quantify the light signal, which is proportional to ATP concentration. We also review a method involving polarographic measurement of oxygen consumption. Measurements of oxygen consumption, which previously required large amounts of tissue, are now feasible with very small amounts of sample obtained by needle biopsy due to recent advances in the field of high-resolution respirometry. We illustrate how careful attention to substrate combinations and inhibitors allows an abundance of unique functional information to be obtained from isolated mitochondria, including function at various energetic states, oxidative capacity with electron flow through distinct complexes, coupling of oxygen consumption to ATP production, and membrane integrity. These measurements, together with studies of mitochondrial DNA abundance, mRNA levels, protein expression, and synthesis rates of mitochondrial proteins provide insightful mechanistic information about mitochondria in a variety of tissue types.
Figures

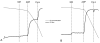

Similar articles
-
Skeletal muscle mitochondria of NDUFS4-/- mice display normal maximal pyruvate oxidation and ATP production.Biochim Biophys Acta. 2015 Jun-Jul;1847(6-7):526-33. doi: 10.1016/j.bbabio.2015.02.006. Epub 2015 Feb 14. Biochim Biophys Acta. 2015. PMID: 25687896
-
High-Resolution FluoRespirometry and OXPHOS Protocols for Human Cells, Permeabilized Fibers from Small Biopsies of Muscle, and Isolated Mitochondria.Methods Mol Biol. 2018;1782:31-70. doi: 10.1007/978-1-4939-7831-1_3. Methods Mol Biol. 2018. PMID: 29850993
-
Comparison of in vivo postexercise phosphocreatine recovery and resting ATP synthesis flux for the assessment of skeletal muscle mitochondrial function.Am J Physiol Cell Physiol. 2010 Nov;299(5):C1136-43. doi: 10.1152/ajpcell.00200.2010. Epub 2010 Jul 28. Am J Physiol Cell Physiol. 2010. PMID: 20668212
-
Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology.Int J Biochem Cell Biol. 2009 Oct;41(10):1837-45. doi: 10.1016/j.biocel.2009.03.013. Epub 2009 Apr 2. Int J Biochem Cell Biol. 2009. PMID: 19467914 Review.
-
Evaluation of in vivo mitochondrial bioenergetics in skeletal muscle using NMR and optical methods.Biochim Biophys Acta. 2016 Apr;1862(4):716-724. doi: 10.1016/j.bbadis.2015.12.019. Epub 2015 Dec 17. Biochim Biophys Acta. 2016. PMID: 26708941 Free PMC article. Review.
Cited by
-
Association of UCP-3 rs1626521 with obesity and stomach functions in humans.Obesity (Silver Spring). 2015 Apr;23(4):898-906. doi: 10.1002/oby.21039. Epub 2015 Mar 7. Obesity (Silver Spring). 2015. PMID: 25755013 Free PMC article.
-
Acquisition of chemoresistance in gliomas is associated with increased mitochondrial coupling and decreased ROS production.PLoS One. 2011;6(9):e24665. doi: 10.1371/journal.pone.0024665. Epub 2011 Sep 9. PLoS One. 2011. PMID: 21931801 Free PMC article.
-
Mitochondrial dysfunction and the pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS).Int J Clin Exp Med. 2012;5(3):208-20. Epub 2012 Jun 15. Int J Clin Exp Med. 2012. PMID: 22837795 Free PMC article.
-
The relationship between mitochondrial respiration, resting metabolic rate and blood cell count in great tits.Biol Open. 2024 Mar 1;13(3):bio060302. doi: 10.1242/bio.060302. Epub 2024 Mar 11. Biol Open. 2024. PMID: 38385271 Free PMC article.
-
Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis.Cell Metab. 2012 Dec 5;16(6):777-88. doi: 10.1016/j.cmet.2012.11.003. Cell Metab. 2012. PMID: 23217257 Free PMC article.
References
-
- Argov Z, Bank WJ, Maris J, Peterson P, Chance B. Bioenergetic heterogeneity of human mitochondrial myopathies: phosphorus magnetic resonance spectroscopy study. Neurology. 1987;37:257–262. - PubMed
-
- Asmann YW, Stump CS, Short KR, Coenen-Schimke JM, Guo Z, Bigelow ML, Nair KS. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes. 2006;55:3309–3319. - PubMed
-
- Chance B, Williams G. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955;217:383–393. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

