Hypoxia upregulates angiogenesis and synovial cell migration in rheumatoid arthritis
- PMID: 19426483
- PMCID: PMC2714109
- DOI: 10.1186/ar2689
Hypoxia upregulates angiogenesis and synovial cell migration in rheumatoid arthritis
Abstract
Introduction: Rheumatoid arthritis (RA) is characterised by invasion of cartilage, bone and tendon by inflamed synovium. Previous studies in our laboratory have shown that hypoxia is a feature of RA synovitis. In the present study, we investigated the consequences of hypoxia on angiogenesis and synovial fibroblast migration in RA.
Methods: Synovial tissue was harvested from RA patients, and synovial membrane cells were cultured under conditions either of hypoxia (1% oxygen) or normoxia (21% oxygen). Protein levels of matrix metalloproteinases (MMPs) and angiogenic factors were measured, while RNA was extracted for PCR quantification of MMPs/tissue inhibitors of MMP (TIMPs) and angiogenic factors. Migration of RA synovial fibroblasts through collagen, and the effect of RA synovial cell supernatants in an in vitro angiogenesis assay, were utilised to determine the functional relevance of changes in mRNA/protein.
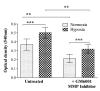
Results: We observed upregulation under hypoxic conditions of MMPs responsible for collagen breakdown, specifically collagenase MMP-8, and the gelatinases MMP-2 and MMP-9, at both mRNA and protein levels. Increased MT1-MMP mRNA was also observed, but no effect on TIMP-1 or TIMP-2 was detected. RA fibroblast migration across collagen was significantly increased under hypoxic conditions, and was dependent on MMP activity. Furthermore, expression of angiogenic stimuli, such as vascular endothelial growth factor (VEGF), and VEGF/placental growth factor heterodimer, was also increased. Crucially, we show for the first time that hypoxia increased the angiogenic drive of RA cells, as demonstrated by enhanced blood vessel formation in an in vitro angiogenesis assay.
Conclusions: Hypoxia may be responsible for rendering RA synovial lining proangiogenic and proinvasive, thus leading to the debilitating features characteristic of RA.
Figures




Similar articles
-
Membrane type 1 matrix metalloproteinase is a crucial promoter of synovial invasion in human rheumatoid arthritis.Arthritis Rheum. 2009 Mar;60(3):686-97. doi: 10.1002/art.24331. Arthritis Rheum. 2009. PMID: 19248098 Free PMC article.
-
Oncostatin M induces angiogenesis and cartilage degradation in rheumatoid arthritis synovial tissue and human cartilage cocultures.Arthritis Rheum. 2006 Oct;54(10):3152-62. doi: 10.1002/art.22161. Arthritis Rheum. 2006. PMID: 17009243
-
Enhanced angiogenic function in response to fibroblasts from psoriatic arthritis synovium compared to rheumatoid arthritis.Arthritis Res Ther. 2019 Dec 21;21(1):297. doi: 10.1186/s13075-019-2088-3. Arthritis Res Ther. 2019. PMID: 31864394 Free PMC article.
-
Hypoxia--a key regulator of angiogenesis and inflammation in rheumatoid arthritis.Nat Rev Rheumatol. 2012 Jan 31;8(3):153-62. doi: 10.1038/nrrheum.2011.205. Nat Rev Rheumatol. 2012. PMID: 22293762 Review.
-
The Role of Extracellular Nucleic Acids in Rheumatoid Arthritis.Curr Pharm Biotechnol. 2018;19(15):1182-1188. doi: 10.2174/1389201020666190102150216. Curr Pharm Biotechnol. 2018. PMID: 30605055 Review.
Cited by
-
A narrative review of the role of the Notch signaling pathway in rheumatoid arthritis.Ann Transl Med. 2022 Mar;10(6):371. doi: 10.21037/atm-22-142. Ann Transl Med. 2022. PMID: 35433937 Free PMC article. Review.
-
Biochemical insights into the role of matrix metalloproteinases in regeneration: challenges and recent developments.Future Med Chem. 2009 Sep;1(6):1095-1111. doi: 10.4155/fmc.09.83. Future Med Chem. 2009. PMID: 20161478 Free PMC article. Review.
-
Elucidating the role of hypoxia-inducible factor in rheumatoid arthritis.Inflammopharmacology. 2022 Jun;30(3):737-748. doi: 10.1007/s10787-022-00974-4. Epub 2022 Apr 1. Inflammopharmacology. 2022. PMID: 35364736 Review.
-
HIF-1α dependent RhoA as a novel therapeutic target to regulate rheumatoid arthritis fibroblast-like synoviocytes migration in vitro and in vivo.J Orthop Translat. 2023 Jun 6;40:49-57. doi: 10.1016/j.jot.2023.05.004. eCollection 2023 May. J Orthop Translat. 2023. PMID: 37346290 Free PMC article.
-
Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis.Am J Respir Cell Mol Biol. 2015 Nov;53(5):585-600. doi: 10.1165/rcmb.2015-0020TR. Am J Respir Cell Mol Biol. 2015. PMID: 26121236 Free PMC article. Review.
References
-
- Fearon U, Griosios K, Fraser A, Reece R, Emery P, Jones PF, Veale DJ. Angiopoietins, growth factors, and vascular morphology in early arthritis. J Rheumatol. 2003;30:260–268. - PubMed
-
- Larsen H, Akhavani MA, Raatz Y, Paleolog EM. Gene expression studies to investigate disease mechanisms in rheumatoid arthritis: does angiogenesis play a role? Curr Rheumatol Rev. 2007;3:243–251. doi: 10.2174/157339707782408991. - DOI
-
- Ceponis A, Konttinen YT, Imai S, Tamulaitiene M, Li TF, Xu JW, Hietanen J, Santavirta S, Fassbender HG. Synovial lining, endothelial and inflammatory mononuclear cell proliferation in synovial membranes in psoriatic and reactive arthritis: a comparative quantitative morphometric study. Br J Rheumatol. 1998;37:170–178. doi: 10.1093/rheumatology/37.2.170. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

