Inhibition of new vessel growth in mouse model of laser-induced choroidal neovascularization by adiponectin peptide II
- PMID: 19422927
- PMCID: PMC3278218
- DOI: 10.1016/j.cellbi.2009.04.013
Inhibition of new vessel growth in mouse model of laser-induced choroidal neovascularization by adiponectin peptide II
Abstract
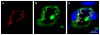
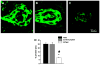
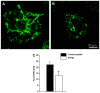
We have investigated the effect of adiponectin (APN) peptide II on new vessel growth in mouse model of choroidal neovascularization (CNV) or wet type age-related macular degeneration (AMD). Mice were injected intraperitoneally with APN peptide II, control peptide, or PBS on day 1-7 or day 5-14. APN, AdipoR1, PCNA, and VEGF localization was investigated using confocal microscopy, immunohistochemistry, and RT-PCR. APN peptide II decreased the relative area of FITC-dextran perfused vessels by 4-fold, PCNA expression by 3-fold, and the number of PCNA stained HUVEC and MAVEC cells by 38 and 46%, respectively. We concluded that APN peptide II inhibits CNV size on days 7 and 14 by inhibiting the proliferation of endothelial cells in vivo and in vitro. APN peptide II may have therapeutic potential to inhibit CNV or wet AMD.
Figures



Similar articles
-
Inhibitory role of adiponectin peptide I on rat choroidal neovascularization.Biochim Biophys Acta. 2012 Aug;1823(8):1264-72. doi: 10.1016/j.bbamcr.2012.05.017. Epub 2012 May 23. Biochim Biophys Acta. 2012. PMID: 22633972 Free PMC article.
-
Expression of adiponectin in choroidal tissue and inhibition of laser induced choroidal neovascularization by adiponectin.FEBS Lett. 2007 May 15;581(10):1977-82. doi: 10.1016/j.febslet.2007.04.024. Epub 2007 Apr 20. FEBS Lett. 2007. PMID: 17466298
-
Emerging Role of Adiponectin/AdipoRs Signaling in Choroidal Neovascularization, Age-Related Macular Degeneration, and Diabetic Retinopathy.Biomolecules. 2023 Jun 13;13(6):982. doi: 10.3390/biom13060982. Biomolecules. 2023. PMID: 37371562 Free PMC article. Review.
-
Adiponectin Mediates Dietary Omega-3 Long-Chain Polyunsaturated Fatty Acid Protection Against Choroidal Neovascularization in Mice.Invest Ophthalmol Vis Sci. 2017 Aug 1;58(10):3862-3870. doi: 10.1167/iovs.17-21796. Invest Ophthalmol Vis Sci. 2017. PMID: 28763559 Free PMC article.
-
Linking Adiponectin and Its Receptors to Age-Related Macular Degeneration (AMD).Biomedicines. 2023 Nov 14;11(11):3044. doi: 10.3390/biomedicines11113044. Biomedicines. 2023. PMID: 38002042 Free PMC article. Review.
Cited by
-
Adiponectin and breast cancer.Med Oncol. 2011 Dec;28(4):1288-95. doi: 10.1007/s12032-010-9617-x. Epub 2010 Jul 13. Med Oncol. 2011. PMID: 20625941 Review.
-
Potential Protective Function of Adiponectin in Diabetic Retinopathy.Ophthalmol Ther. 2023 Jun;12(3):1519-1534. doi: 10.1007/s40123-023-00702-3. Epub 2023 Mar 31. Ophthalmol Ther. 2023. PMID: 37000404 Free PMC article. Review.
-
The effect of nicotine on anti-vascular endothelial growth factor therapy in a mouse model of neovascular age-related macular degeneration.Retina. 2012 Jun;32(6):1171-80. doi: 10.1097/IAE.0b013e31823496b8. Retina. 2012. PMID: 22088983 Free PMC article.
-
Recombinant adiponectin peptide promotes neuronal survival after intracerebral haemorrhage by suppressing mitochondrial and ATF4-CHOP apoptosis pathways in diabetic mice via Smad3 signalling inhibition.Cell Prolif. 2020 Feb;53(2):e12759. doi: 10.1111/cpr.12759. Epub 2020 Jan 10. Cell Prolif. 2020. PMID: 31922310 Free PMC article.
-
Inhibitory role of adiponectin peptide I on rat choroidal neovascularization.Biochim Biophys Acta. 2012 Aug;1823(8):1264-72. doi: 10.1016/j.bbamcr.2012.05.017. Epub 2012 May 23. Biochim Biophys Acta. 2012. PMID: 22633972 Free PMC article.
References
-
- Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–8. - PubMed
-
- Bora PS, Sohn JH, Cruz JM, Jha P, Nishihori H, Wang Y, et al. Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J Immunol. 2005;174:491–7. - PubMed
-
- Bora PS, Kaliappan S, Lyzogubov VV, Tytarenko RG, Thotakura S, Viswanathan T, et al. Expression of adiponectin in choroidal tissue and inhibition of laser-induced choroidal neovascularization by adiponectin. FEBS Lett. 2007;581:1977–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

