Chk1 C-terminal regulatory phosphorylation mediates checkpoint activation by de-repression of Chk1 catalytic activity
- PMID: 19421147
- PMCID: PMC2857325
- DOI: 10.1038/onc.2009.102
Chk1 C-terminal regulatory phosphorylation mediates checkpoint activation by de-repression of Chk1 catalytic activity
Abstract
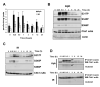
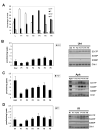
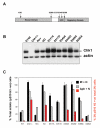
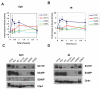
Chk1 is phosphorylated within its C-terminal regulatory domain by the upstream ATM/ATR kinases during checkpoint activation; however, how this modulates Chk1 function is poorly understood. Here, we show that Chk1 kinase activity is rapidly stimulated in a cell-cycle phase-specific manner in response to both DNA damage and replication arrest, and that the extent and duration of activation correlates closely with regulatory phosphorylation at serines (S) S317, S345 and S366. Despite their evident co-regulation, substitutions of individual Chk1 regulatory sites with alanine (A) residues have differential effects on checkpoint proficiency and kinase activation. Thus, whereas Chk1 S345 is essential for all functions tested, mutants lacking S317 or S366 retain partial proficiency for G2/M and S/M checkpoint arrests triggered by DNA damage or replication arrest. These phenotypes reflect defects in Chk1 kinase induction, as the mutants are either partially (317A and 366A) or completely (345A) resistant to kinase activation. Importantly, S345 phosphorylation is impaired in Chk1 S317A and S366A mutants, suggesting that modification of adjacent SQ sites promotes this key regulatory event. Finally, we provide biochemical evidence that Chk1 catalytic activity is stimulated by a de-repression mechanism.
Figures
Similar articles
-
Ataxia-telangiectasia-mutated (ATM) and NBS1-dependent phosphorylation of Chk1 on Ser-317 in response to ionizing radiation.J Biol Chem. 2003 Apr 25;278(17):14806-11. doi: 10.1074/jbc.M210862200. Epub 2003 Feb 14. J Biol Chem. 2003. PMID: 12588868
-
Dissecting cellular responses to irradiation via targeted disruptions of the ATM-CHK1-PP2A circuit.Cell Cycle. 2013 Apr 1;12(7):1105-18. doi: 10.4161/cc.24127. Epub 2013 Mar 5. Cell Cycle. 2013. PMID: 23462183 Free PMC article.
-
Suppression of Tousled-like kinase activity after DNA damage or replication block requires ATM, NBS1 and Chk1.Oncogene. 2003 Sep 4;22(38):5927-37. doi: 10.1038/sj.onc.1206691. Oncogene. 2003. PMID: 12955071
-
Chk1 in the DNA damage response: conserved roles from yeasts to mammals.DNA Repair (Amst). 2004 Aug-Sep;3(8-9):1025-32. doi: 10.1016/j.dnarep.2004.03.003. DNA Repair (Amst). 2004. PMID: 15279789 Review.
-
Mechanisms of ATR-mediated checkpoint signalling.Front Biosci (Landmark Ed). 2010 Jun 1;15(3):840-53. doi: 10.2741/3649. Front Biosci (Landmark Ed). 2010. PMID: 20515729 Review.
Cited by
-
Conformational Change of Human Checkpoint Kinase 1 (Chk1) Induced by DNA Damage.J Biol Chem. 2016 Jun 17;291(25):12951-9. doi: 10.1074/jbc.M115.713248. Epub 2016 Apr 18. J Biol Chem. 2016. PMID: 27129240 Free PMC article.
-
An ATR and CHK1 kinase signaling mechanism that limits origin firing during unperturbed DNA replication.Proc Natl Acad Sci U S A. 2019 Jul 2;116(27):13374-13383. doi: 10.1073/pnas.1903418116. Epub 2019 Jun 17. Proc Natl Acad Sci U S A. 2019. PMID: 31209037 Free PMC article.
-
Targeting the checkpoint kinase Chk1 in cancer therapy.Cell Cycle. 2010 Jan 15;9(2):279-83. doi: 10.4161/cc.9.2.10445. Epub 2010 Jan 27. Cell Cycle. 2010. PMID: 20023404 Free PMC article. Review.
-
Evidence for functional and regulatory cross-talk between Wnt/β-catenin signalling and Mre11-Rad50-Nbs1 complex in the repair of cisplatin-induced DNA cross-links.Oncotarget. 2020 Nov 3;11(44):4028-4044. doi: 10.18632/oncotarget.27777. eCollection 2020 Nov 3. Oncotarget. 2020. PMID: 33216839 Free PMC article.
-
Checkpoint kinase 1 in DNA damage response and cell cycle regulation.Cell Mol Life Sci. 2013 Nov;70(21):4009-21. doi: 10.1007/s00018-013-1307-3. Epub 2013 Mar 19. Cell Mol Life Sci. 2013. PMID: 23508805 Free PMC article. Review.
References
-
- Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. - PubMed
-
- Chen P, Luo C, Deng Y, Ryan K, Register J, Margosiak S, Tempczyk-Russell A, Nguyen B, Myers P, Lundgren K, Kan CC, O’Connor PM. The 1.7 A crystal structure of human cell cycle checkpoint kinase Chk1: implications for Chk1 regulation. Cell. 2000;100:681–692. - PubMed
-
- Chen Y, Sanchez Y. Chk1 in the DNA damage response: conserved roles from yeasts to mammals. DNA Repair (Amst) 2004;3:1025–1032. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

