Molecular recognition of the palmitoylation substrate Vac8 by its palmitoyltransferase Pfa3
- PMID: 19416974
- PMCID: PMC2719411
- DOI: 10.1074/jbc.M109.005447
Molecular recognition of the palmitoylation substrate Vac8 by its palmitoyltransferase Pfa3
Abstract
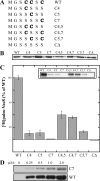
Palmitoylation of the yeast vacuolar protein Vac8 is important for its role in membrane-mediated events such as vacuole fusion. It has been established both in vivo and in vitro that Vac8 is palmitoylated by the Asp-His-His-Cys (DHHC) protein Pfa3. However, the determinants of Vac8 critical for recognition by Pfa3 have yet to be elucidated. This is of particular importance because of the lack of a consensus sequence for palmitoylation. Here we show that Pfa3 was capable of palmitoylating each of the three N-terminal cysteines of Vac8 and that this reaction was most efficient when Vac8 is N-myristoylated. Additionally, when we analyzed the Src homology 4 (SH4) domain of Vac8 independent of the rest of the protein, palmitoylation by Pfa3 still occurred. However, the specificity of palmitoylation seen for the full-length protein was lost, and the SH4 domain was palmitoylated by all five of the yeast DHHC proteins tested. These data suggested that a region of the protein C-terminal to the SH4 domain was important for conferring specificity of palmitoylation. This was confirmed by use of a chimeric protein in which the SH4 domain of Vac8 was swapped for that of Meh1, another palmitoylated and N-myristoylated protein in yeast. In this case we saw specificity mimic that of wild type Vac8. Competition experiments revealed that the 11th armadillo repeat of Vac8 is an important element for recognition by Pfa3. This demonstrates that regions distant from the palmitoylated cysteines are important for recognition by DHHC proteins.
Figures
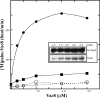


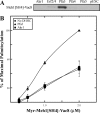

 ), myr-Vac8[Arm1–10](CΔ)-myc-6xHIS (♢), or myr-Vac8[ArmΔ11](CΔ)-myc-6xHIS (□) were incubated with 0.1 μ
), myr-Vac8[Arm1–10](CΔ)-myc-6xHIS (♢), or myr-Vac8[ArmΔ11](CΔ)-myc-6xHIS (□) were incubated with 0.1 μSimilar articles
-
The DHHC protein Pfa3 affects vacuole-associated palmitoylation of the fusion factor Vac8.Proc Natl Acad Sci U S A. 2005 Nov 29;102(48):17366-71. doi: 10.1073/pnas.0508885102. Epub 2005 Nov 21. Proc Natl Acad Sci U S A. 2005. PMID: 16301533 Free PMC article.
-
Analysis of DHHC acyltransferases implies overlapping substrate specificity and a two-step reaction mechanism.Traffic. 2009 Aug;10(8):1061-73. doi: 10.1111/j.1600-0854.2009.00925.x. Epub 2009 May 12. Traffic. 2009. PMID: 19453970
-
Palmitoylation determines the function of Vac8 at the yeast vacuole.J Cell Sci. 2006 Jun 15;119(Pt 12):2477-85. doi: 10.1242/jcs.02972. Epub 2006 May 23. J Cell Sci. 2006. PMID: 16720644
-
Probing protein palmitoylation at the yeast vacuole.Methods. 2006 Oct;40(2):171-6. doi: 10.1016/j.ymeth.2006.06.020. Methods. 2006. PMID: 17012029 Review.
-
Protein palmitoylation by a family of DHHC protein S-acyltransferases.J Lipid Res. 2006 Jun;47(6):1118-27. doi: 10.1194/jlr.R600007-JLR200. Epub 2006 Apr 1. J Lipid Res. 2006. PMID: 16582420 Review.
Cited by
-
The physiology of protein S-acylation.Physiol Rev. 2015 Apr;95(2):341-76. doi: 10.1152/physrev.00032.2014. Physiol Rev. 2015. PMID: 25834228 Free PMC article. Review.
-
Distinct palmitoylation events at the amino-terminal conserved cysteines of Env7 direct its stability, localization, and vacuolar fusion regulation in S. cerevisiae.J Biol Chem. 2014 Apr 18;289(16):11431-11442. doi: 10.1074/jbc.M113.524082. Epub 2014 Mar 7. J Biol Chem. 2014. PMID: 24610781 Free PMC article.
-
An electrostatic switch controls palmitoylation of the large conductance voltage- and calcium-activated potassium (BK) channel.J Biol Chem. 2012 Jan 6;287(2):1468-77. doi: 10.1074/jbc.M111.224840. Epub 2011 Nov 14. J Biol Chem. 2012. PMID: 22084244 Free PMC article.
-
Regulation of Sensing, Transportation, and Catabolism of Nitrogen Sources in Saccharomyces cerevisiae.Microbiol Mol Biol Rev. 2018 Feb 7;82(1):e00040-17. doi: 10.1128/MMBR.00040-17. Print 2018 Jun. Microbiol Mol Biol Rev. 2018. PMID: 29436478 Free PMC article. Review.
-
Dynamic palmitoylation and the role of DHHC proteins in T cell activation and anergy.Adv Immunol. 2011;109:1-44. doi: 10.1016/B978-0-12-387664-5.00001-7. Adv Immunol. 2011. PMID: 21569911 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

