SUMO interaction motifs in Sizn1 are required for promyelocytic leukemia protein nuclear body localization and for transcriptional activation
- PMID: 19416967
- PMCID: PMC2740585
- DOI: 10.1074/jbc.M109.010181
SUMO interaction motifs in Sizn1 are required for promyelocytic leukemia protein nuclear body localization and for transcriptional activation
Abstract
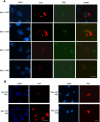
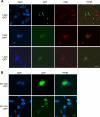
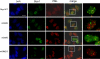
Mutations in Sizn1 (Zcchc12), a novel transcriptional co-activator in the BMP signaling pathway, are associated with X-linked mental retardation. Previously, we demonstrated that Sizn1 positively modulates the BMP signal by interacting with Smad family members and cAMP-responsive element-binding protein-binding protein. To further define the molecular basis of Sizn1 function, we have explored its subcellular localization and generated various deletion mutants to carry out domain analyses. Here, we report that Sizn1 localizes to promyelocytic leukemia protein nuclear bodies (PML-NBs). Sizn1 deletion mutants that disrupt the MA homologous domain or the middle region fail to target to the PML-NB. We show that two SUMO interaction motifs (SIMs) in Sizn1 can bind to SUMO and govern SUMO conjugation to Sizn1 in the absence of the consensus motif for SUMO attachment. Interestingly, the SIM mutant Sizn1 localizes to nuclear bodies, but not to PML-NBs. Thus, SIMs mediate the localization of Sizn1 to PML-NB. Interestingly, mutations in SIM sequences and deletion of the MA homologous domain also affected the transcriptional co-activation function of a Sizn1. Taken together, our data indicate that the SIMs in Sizn1 are required for its PML-NB localization and for the full transcriptional co-activation function in BMP signaling.
Figures




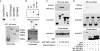
Similar articles
-
Stabilization of PML nuclear localization by conjugation and oligomerization of SUMO-3.Oncogene. 2005 Aug 18;24(35):5401-13. doi: 10.1038/sj.onc.1208714. Oncogene. 2005. PMID: 15940266
-
Disruption of PML nuclear bodies is mediated by ORF61 SUMO-interacting motifs and required for varicella-zoster virus pathogenesis in skin.PLoS Pathog. 2011 Aug;7(8):e1002157. doi: 10.1371/journal.ppat.1002157. Epub 2011 Aug 25. PLoS Pathog. 2011. PMID: 21901090 Free PMC article.
-
Role of SUMO in RNF4-mediated promyelocytic leukemia protein (PML) degradation: sumoylation of PML and phospho-switch control of its SUMO binding domain dissected in living cells.J Biol Chem. 2009 Jun 12;284(24):16595-16608. doi: 10.1074/jbc.M109.006387. Epub 2009 Apr 20. J Biol Chem. 2009. PMID: 19380586 Free PMC article.
-
Role of nuclear bodies in apoptosis signalling.Biochim Biophys Acta. 2008 Nov;1783(11):2185-94. doi: 10.1016/j.bbamcr.2008.07.002. Epub 2008 Jul 16. Biochim Biophys Acta. 2008. PMID: 18680765 Review.
-
A Tale of Usurpation and Subversion: SUMO-Dependent Integrity of Promyelocytic Leukemia Nuclear Bodies at the Crossroad of Infection and Immunity.Front Cell Dev Biol. 2021 Aug 27;9:696234. doi: 10.3389/fcell.2021.696234. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34513832 Free PMC article. Review.
Cited by
-
Characterization of selective ubiquitin and ubiquitin-like protease inhibitors using a fluorescence-based multiplex assay format.Assay Drug Dev Technol. 2011 Apr;9(2):165-73. doi: 10.1089/adt.2010.0317. Epub 2010 Dec 6. Assay Drug Dev Technol. 2011. PMID: 21133675 Free PMC article.
-
Zinc controls PML nuclear body formation through regulation of a paralog specific auto-inhibition in SUMO1.Nucleic Acids Res. 2022 Aug 12;50(14):8331-8348. doi: 10.1093/nar/gkac620. Nucleic Acids Res. 2022. PMID: 35871297 Free PMC article.
-
ZCCHC12 promotes the progression of osteosarcoma via PI3K/AKT pathway.Aging (Albany NY). 2022 Sep 19;14(18):7505-7516. doi: 10.18632/aging.204296. Epub 2022 Sep 19. Aging (Albany NY). 2022. PMID: 36126191 Free PMC article.
-
A novel SUMO1-specific interacting motif in dipeptidyl peptidase 9 (DPP9) that is important for enzymatic regulation.J Biol Chem. 2012 Dec 28;287(53):44320-9. doi: 10.1074/jbc.M112.397224. Epub 2012 Nov 14. J Biol Chem. 2012. PMID: 23152501 Free PMC article.
-
SUMOylation of DEC1 protein regulates its transcriptional activity and enhances its stability.PLoS One. 2011;6(8):e23046. doi: 10.1371/journal.pone.0023046. Epub 2011 Aug 1. PLoS One. 2011. PMID: 21829689 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

