Regulation of the formation and trafficking of vesicles from Golgi by PCH family proteins during chemotaxis
- PMID: 19409937
- PMCID: PMC2703453
- DOI: 10.1016/j.bbamcr.2009.04.012
Regulation of the formation and trafficking of vesicles from Golgi by PCH family proteins during chemotaxis
Abstract
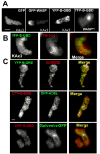
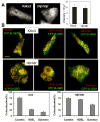
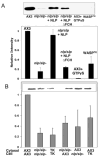
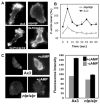
Previous study demonstrated that WASP localizes on vesicles during Dictyostelium chemotaxis and these vesicles appear to be preferentially distributed at the leading and trailing edge of migrating cells. In this study, we have examined the role of PCH family proteins, Nwk/Bzz1p-like protein (NLP) and Syndapin-like protein (SLP), in the regulation of the formation and trafficking of WASP-vesicles during chemotaxis. NLP and SLP appear to be functionally redundant and deletion of both nlp and slp genes causes the loss of polarized F-actin organization and significant defects in chemotaxis. WASP and NLP are colocalized on vesicles and interactions between two molecules via the SH3 domain of NLP/SLP and the proline-rich repeats of WASP are required for vesicle formation from Golgi. Microtubules are required for polarized trafficking of these vesicles as vesicles showing high directed mobility are absent in cells treated with nocodazole. Our results suggest that interaction of WASP with NLP/SLP is required for the formation and trafficking of vesicles from Golgi to the membrane, which might play a central role in the establishment of cell polarity during chemotaxis.
Figures


Similar articles
-
A Dictyostelium homologue of WASP is required for polarized F-actin assembly during chemotaxis.Mol Biol Cell. 2005 May;16(5):2191-206. doi: 10.1091/mbc.e04-09-0844. Epub 2005 Feb 23. Mol Biol Cell. 2005. PMID: 15728724 Free PMC article.
-
Role of RacC for the regulation of WASP and phosphatidylinositol 3-kinase during chemotaxis of Dictyostelium.J Biol Chem. 2006 Nov 17;281(46):35224-34. doi: 10.1074/jbc.M605997200. Epub 2006 Sep 12. J Biol Chem. 2006. PMID: 16968699 Free PMC article.
-
An attenuating role of a WASP-related protein, WASP-B, in the regulation of F-actin polymerization and pseudopod formation via the regulation of RacC during Dictyostelium chemotaxis.Biochem Biophys Res Commun. 2013 Jul 12;436(4):719-24. doi: 10.1016/j.bbrc.2013.06.022. Epub 2013 Jun 17. Biochem Biophys Res Commun. 2013. PMID: 23791739 Free PMC article.
-
The syndapin protein family: linking membrane trafficking with the cytoskeleton.J Cell Sci. 2004 Jul 1;117(Pt 15):3077-86. doi: 10.1242/jcs.01290. J Cell Sci. 2004. PMID: 15226389 Review.
-
Cdc42 and vesicle trafficking in polarized cells.Traffic. 2010 Oct;11(10):1272-9. doi: 10.1111/j.1600-0854.2010.01102.x. Traffic. 2010. PMID: 20633244 Review.
Cited by
-
Myosin I links PIP3 signaling to remodeling of the actin cytoskeleton in chemotaxis.Sci Signal. 2012 Jan 31;5(209):ra10. doi: 10.1126/scisignal.2002446. Sci Signal. 2012. PMID: 22296834 Free PMC article.
-
Microtubule segment stabilization by RASSF1A is required for proper microtubule dynamics and Golgi integrity.Mol Biol Cell. 2014 Mar;25(6):800-10. doi: 10.1091/mbc.E13-07-0374. Epub 2014 Jan 29. Mol Biol Cell. 2014. PMID: 24478455 Free PMC article.
-
Actin acting at the Golgi.Histochem Cell Biol. 2013 Sep;140(3):347-60. doi: 10.1007/s00418-013-1115-8. Epub 2013 Jun 27. Histochem Cell Biol. 2013. PMID: 23807268 Review.
-
Microtopographical guidance of macropinocytic signaling patches.Proc Natl Acad Sci U S A. 2021 Dec 14;118(50):e2110281118. doi: 10.1073/pnas.2110281118. Proc Natl Acad Sci U S A. 2021. PMID: 34876521 Free PMC article.
References
-
- Locker J, Goldblatt PJ, Leighton J. Ultrastructural features of invasion in chick embryo liver metastasis of Yoshida ascites hepatoma. Cancer Res. 1970;30:1632–1644. - PubMed
-
- Lahrtz F, Piali L, Spanaus KS, Seebach J, Fontana A. Chemokines and chemotaxis of leukocytes in infectious meningitis. J Neuroimmunol. 1998;85:33–43. - PubMed
-
- Downey GP. Mechanisms of leukocyte motility and chemotaxis. Curr Opin Immunol. 1994;6:113–124. - PubMed
-
- Devreotes PN, Zigmond SH. Chemotaxis in eukaryotic cells: A focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. - PubMed
-
- Symons M, Derry JM, Karlak B, Jiang S, Lemahieu V, Mccormick F, Francke U, Abo A. Wiskott-Aldrich syndrome protein, a novel effector for the GTPase CDC42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

