QLT0267, a small molecule inhibitor targeting integrin-linked kinase (ILK), and docetaxel can combine to produce synergistic interactions linked to enhanced cytotoxicity, reductions in P-AKT levels, altered F-actin architecture and improved treatment outcomes in an orthotopic breast cancer model
- PMID: 19409087
- PMCID: PMC2716491
- DOI: 10.1186/bcr2252
QLT0267, a small molecule inhibitor targeting integrin-linked kinase (ILK), and docetaxel can combine to produce synergistic interactions linked to enhanced cytotoxicity, reductions in P-AKT levels, altered F-actin architecture and improved treatment outcomes in an orthotopic breast cancer model
Abstract
Introduction: Substantial preclinical evidence has indicated that inhibition of integrin linked-kinase (ILK) correlates with cytotoxic/cytostatic cellular effects, delayed tumor growth in animal models of cancer, and inhibition of angiogenesis. Widely anticipated to represent a very promising therapeutic target in several cancer indications, it is increasingly evident that optimal therapeutic benefits obtained using ILK targeting strategies will only be achieved in combination settings. The purpose of this study was to investigate the therapeutic potential of the ILK small molecule inhibitor, QLT0267 (267), alone or in combination with chemotherapies commonly used to treat breast cancer patients.
Methods: A single end-point metabolic assay was used as an initial screen for 267 interactions with selected chemotherapeutic agents. These in vitro assays were completed with seven breast cancer cell lines including several which over-expressed human epidermal growth factor receptor 2 (Her2). One agent, docetaxel (Dt), consistently produced synergistic interactions when combined with 267. Dt/267 interactions were further characterized by measuring therapeutic endpoints linked to phosphorylated protein kinase B (P-AKT) suppression, inhibition of vascular endothelial growth factor (VEGF) secretion and changes in cytoarchitecture. In vivo efficacy studies were completed in mice bearing orthotopic xenografts where tumor growth was assessed by bioluminescence and calliper methods.
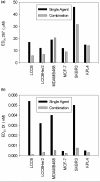
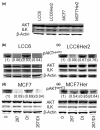
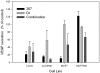
Results: The combination of 267 and Dt resulted in increased cytotoxic activity, as determined using an assay of metabolic activity. Combinations of cisplatin, doxorubicin, vinorelbine, paclitaxel, and trastuzumab produced antagonistic interactions. Further endpoint analysis in cell lines with low Her2 levels revealed that the 267/Dt combinations resulted in: a three-fold decrease in concentration (dose) of 267 required to achieve 50% inhibition of P-AKT; and a dramatic disruption of normal filamentous-actin cellular architecture. In contrast to Her2-positive cell lines, three-fold higher concentrations of 267 were required to achieve 50% inhibition of P-AKT when the drug was used in combination with Dt. In vivo studies focusing on low Her2-expressing breast cancer cells (LCC6) implanted orthotopically demonstrated that treatment with 267/Dt engendered improved therapeutic effects when compared with mice treated with either agent alone.
Conclusions: The findings indicate that the 267/Dt drug combination confers increased (synergistic) therapeutic efficacy towards human breast cancer cells that express low levels of Her2.
Figures
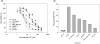



Similar articles
-
Validating the use of a luciferase labeled breast cancer cell line, MDA435LCC6, as a means to monitor tumor progression and to assess the therapeutic activity of an established anticancer drug, docetaxel (Dt) alone or in combination with the ILK inhibitor, QLT0267.Cancer Biol Ther. 2011 May 1;11(9):826-38. doi: 10.4161/cbt.11.9.15183. Epub 2011 May 1. Cancer Biol Ther. 2011. PMID: 21358264 Free PMC article.
-
Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer.J Natl Cancer Inst. 2004 May 19;96(10):739-49. doi: 10.1093/jnci/djh131. J Natl Cancer Inst. 2004. PMID: 15150302
-
Suppression of VEGF secretion and changes in glioblastoma multiforme microenvironment by inhibition of integrin-linked kinase (ILK).Mol Cancer Ther. 2008 Jan;7(1):59-70. doi: 10.1158/1535-7163.MCT-07-0329. Mol Cancer Ther. 2008. PMID: 18202010
-
Rationale for trastuzumab (Herceptin) in adjuvant breast cancer trials.Semin Oncol. 2001 Feb;28(1 Suppl 3):13-9. doi: 10.1016/s0093-7754(01)90188-5. Semin Oncol. 2001. PMID: 11301370 Review.
-
Docetaxel in combination chemotherapy for metastatic breast cancer.Semin Oncol. 1997 Aug;24(4 Suppl 13):S13-19-S13-26. Semin Oncol. 1997. PMID: 9335513 Review.
Cited by
-
Extranuclear signaling by estrogen: role in breast cancer progression and metastasis.Minerva Ginecol. 2010 Dec;62(6):573-83. Minerva Ginecol. 2010. PMID: 21079578 Free PMC article. Review.
-
Integrin-linked kinase (ILK): the known vs. the unknown and perspectives.Cell Mol Life Sci. 2022 Jan 28;79(2):100. doi: 10.1007/s00018-021-04104-1. Cell Mol Life Sci. 2022. PMID: 35089438 Free PMC article. Review.
-
Anaphase catastrophe is a target for cancer therapy.Clin Cancer Res. 2011 Mar 15;17(6):1218-22. doi: 10.1158/1078-0432.CCR-10-1178. Epub 2011 Feb 2. Clin Cancer Res. 2011. PMID: 21288923 Free PMC article.
-
Loss of Integrin-Linked Kinase Sensitizes Breast Cancer to SRC Inhibitors.Cancer Res. 2022 Feb 15;82(4):632-647. doi: 10.1158/0008-5472.CAN-21-0373. Cancer Res. 2022. PMID: 34921014 Free PMC article.
-
Integrin-linked kinase activity modulates the pro-metastatic behavior of ovarian cancer cells.Oncotarget. 2016 Apr 19;7(16):21968-81. doi: 10.18632/oncotarget.7880. Oncotarget. 2016. PMID: 26959113 Free PMC article.
References
-
- Yau CY, Wheeler JJ, Sutton KL, Hedley DW. Inhibition of integrin-linked kinase by a selective small molecule inhibitor, QLT inhibits the PI3K/PKB/mTOR, Stat3, and FKHR pathways and tumor growth, and enhances gemcitabine-induced apoptosis in human orthotopic primary pancreatic cancer xenografts. Cancer Res. 2005;65:1497–1504. doi: 10.1158/0008-5472.CAN-04-2940. - DOI - PubMed
-
- Wu C. Integrin-linked kinase and PINCH: partners in regulation of cell-extracellular matrix interaction and signal transduction. J Cell Sci. 1999;112(Pt 24):4485–4489. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

