Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice
- PMID: 19407337
- PMCID: PMC2696329
- DOI: 10.1093/toxsci/kfp094
Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice
Abstract
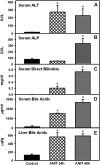
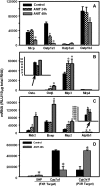
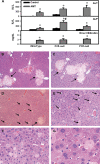
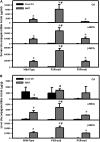
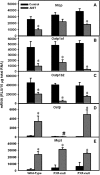
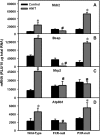
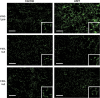
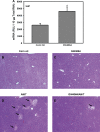
Alpha-naphthyl isothiocyanate (ANIT) is a hepatotoxicant that produces acute intrahepatic cholestasis in rodents. Farnesoid X receptor (FXR) and pregnane X receptor (PXR) are two major bile acid sensors in liver. The purpose of this study was to characterize the regulation of hepatic transporters by FXR and PXR during ANIT-induced liver injury. Wild-type, FXR-null, and PXR-null mice were administered ANIT (75 mg/kg, po) and evaluated 48 h later for hepatotoxicity and messenger RNA (mRNA) expression of basolateral uptake (sodium taurocholate-cotransporting polypeptide, organic anion transporting polypeptide [Oatp] 1a1, Oatp1a4, Oatp1b2) and efflux transporters (organic solute transporter [Ost] alpha, Ostbeta, multidrug resistance-associated protein [Mrp] 3, Mrp4), as well as canalicular transporters (bile salt export pump [Bsep], Mrp2, multidrug resistance protein 2 [Mdr2], ATPase, class I, type 8B, member 1 [Atp8b1]). Livers from wild-type and PXR-null mice had comparable multifocal necrosis 48 h after ANIT. However, ANIT-treated FXR-null mice have fewer and smaller necrotic foci than wild-type mice but had scattered single-cell hepatocyte necrosis throughout the liver. Serum alanine transaminase, alkaline phosphatase (ALP), and direct bilirubin were increased in all genotypes, with higher ALP levels in FXR-null mice. Serum and liver unconjugated bile acids were higher in ANIT-treated FXR-null mice than the other two genotypes. ANIT induced mRNA expression of Mdr2, Bsep, and Atp8b1 in wild-type and PXR-null mice but failed to upregulate these genes in FXR-null mice. mRNA expression of uptake transporters declined in livers of all genotypes following ANIT treatment. ANIT increased Ostbeta and Mrp3 mRNA in livers of wild-type and PXR-null mice but did not alter Ostbeta mRNA in FXR-null mice. In conclusion, FXR deficiency enhances susceptibility of mice to ANIT-induced liver injury, likely a result of impaired induction of hepatobiliary efflux transporters and subsequent hepatic accumulation of unconjugated bile acids.
Figures

Similar articles
-
ANIT-induced intrahepatic cholestasis alters hepatobiliary transporter expression via Nrf2-dependent and independent signaling.Toxicol Sci. 2009 Apr;108(2):247-57. doi: 10.1093/toxsci/kfp020. Epub 2009 Jan 29. Toxicol Sci. 2009. PMID: 19181614 Free PMC article.
-
Alisol B 23-acetate protects against ANIT-induced hepatotoxity and cholestasis, due to FXR-mediated regulation of transporters and enzymes involved in bile acid homeostasis.Toxicol Appl Pharmacol. 2015 Mar 15;283(3):178-86. doi: 10.1016/j.taap.2015.01.020. Epub 2015 Feb 3. Toxicol Appl Pharmacol. 2015. PMID: 25655198
-
Geniposidic acid protected against ANIT-induced hepatotoxity and acute intrahepatic cholestasis, due to Fxr-mediated regulation of Bsep and Mrp2.J Ethnopharmacol. 2016 Feb 17;179:197-207. doi: 10.1016/j.jep.2015.12.033. Epub 2015 Dec 23. J Ethnopharmacol. 2016. PMID: 26723467
-
Bile salt excretory pump: biology and pathobiology.J Pediatr Gastroenterol Nutr. 2006 Jul;43 Suppl 1:S10-6. doi: 10.1097/01.mpg.0000226385.71859.5f. J Pediatr Gastroenterol Nutr. 2006. PMID: 16819395 Review.
-
Hepatobiliary transport of bile acids and organic anions.J Hepatobiliary Pancreat Surg. 2002;9(4):443-7. doi: 10.1007/s005340200055. J Hepatobiliary Pancreat Surg. 2002. PMID: 12483266 Review.
Cited by
-
Changes of organic anion transporter MRP4 and related nuclear receptors in human obstructive cholestasis.J Gastrointest Surg. 2011 Jun;15(6):996-1004. doi: 10.1007/s11605-011-1473-2. Epub 2011 Feb 26. J Gastrointest Surg. 2011. PMID: 21359593
-
Total Iridoid Glycosides from Swertia mussotii Franch. Alleviate Cholestasis Induced by α-Naphthyl Isothiocyanate through Activating the Farnesoid X Receptor and Inhibiting Oxidative Stress.Int J Mol Sci. 2024 Oct 2;25(19):10607. doi: 10.3390/ijms251910607. Int J Mol Sci. 2024. PMID: 39408937 Free PMC article.
-
A Possible Mechanism of Metformin in Improving Insulin Resistance in Diabetic Rat Models.Int J Endocrinol. 2019 Oct 13;2019:3248527. doi: 10.1155/2019/3248527. eCollection 2019. Int J Endocrinol. 2019. PMID: 31737069 Free PMC article.
-
Regulation of hepatic ABCC transporters by xenobiotics and in disease states.Drug Metab Rev. 2010 Aug;42(3):482-538. doi: 10.3109/03602531003654915. Drug Metab Rev. 2010. PMID: 20233023 Free PMC article. Review.
-
Possible Role of Extracellular Vesicles in Hepatotoxicity of Acetaminophen.Int J Mol Sci. 2022 Aug 9;23(16):8870. doi: 10.3390/ijms23168870. Int J Mol Sci. 2022. PMID: 36012131 Free PMC article.
References
-
- Aleksunes LM, Slitt AM, Cherrington NJ, Thibodeau MS, Klaassen CD, Manautou JE. Differential expression of mouse hepatic transporter genes in response to acetaminophen and carbon tetrachloride. Toxicol. Sci. 2005;83(1):44–52. - PubMed
-
- Alvarez L, Jara P, Sanchez-Sabate E, Hierro L, Larrauri J, Diaz MC, Camarena C, De la Vega A, Frauca E, Lopez-Collazo E, et al. Reduced hepatic expression of farnesoid X receptor in hereditary cholestasis associated to mutation in ATP8B1. Hum. Mol. Genet. 2004;13(20):2451–2460. - PubMed
-
- Ballatori N, Christian WV, Lee JY, Dawson PA, Soroka CJ, Boyer JL, Madejczyk MS, Li N. OSTalpha-OSTbeta: A major basolateral bile acid and steroid transporter in human intestinal, renal, and biliary epithelia. Hepatology. 2005;42(6):1270–1279. - PubMed
-
- Burdelski M, Rogiers X. Liver transplantation in metabolic disorders. Acta Gastroenterol. Belg. 1999;62(3):300–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

